We live in an invisible sea of gases that make up the our rather thin but life sustaining atmosphere. Many of the physical properties of gases such as the relationships between their pressure, volume and temperature were investigated by amateur but enthusiastic scientists using rather crude apparatus in the 17th-19th centuries. Through a series of simple but ingenious experiments they were able to derive a set of laws linking pressure, temperature, volume and amount of gas present. These laws are called the gas laws.
Robert Boyle was a scientist who believed in investigating science through experimental methods.
He carried out the experiment shown below to investigate the relationship between the pressure
and volume of a gas.
Here some simple glass u-tubes were filled with liquid mercury. The tubes were sealed at one end.
He measured how the volume of trapped gas
changed as he added mercury columns of various heights.
Throughout his experiments the temperature and
amount of gas present were kept constant.

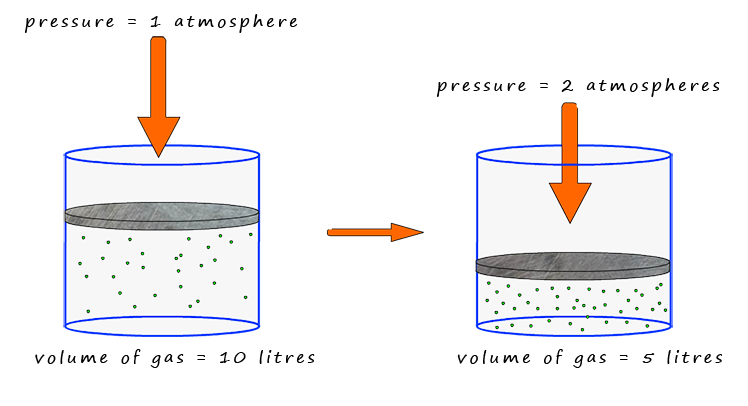
What Boyle found was that as the pressure increased the volume decreased e.g. as the pressure was double the volume halved , or if the volume of the gas increases by a factor of 5, then the pressure has decreased to a 1/5 of what it was previously. This means that the volume of the gas is inversely proportional to the pressure. The image below summarises the relationship between the pressure and volume of a gas.
This means that the pressure p, multiplied by the volume V is always a constant.
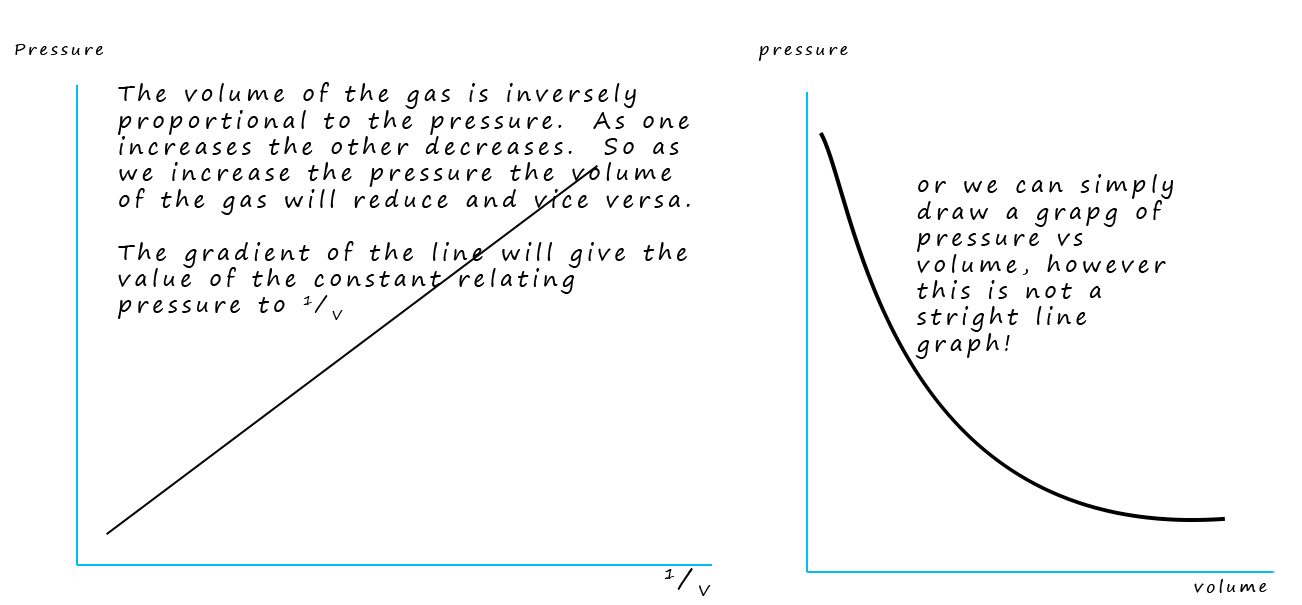
The value of the constant k depends on the temperature and the amount of gas present (number of moles of gas) . However if a graph of V versus 1/p is plotted then the gradient of the line produced will provide the value of k.
As the temperature(T) of a fixed mass (n) of gas at constant pressure (p) increases it expands in a linear fashion. That is:
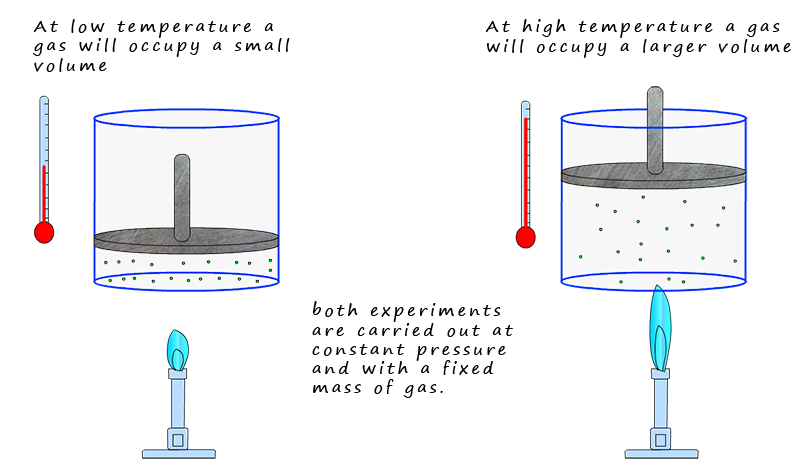
 By taking many volume and temperature reading the following is found:
By taking many volume and temperature reading the following is found:
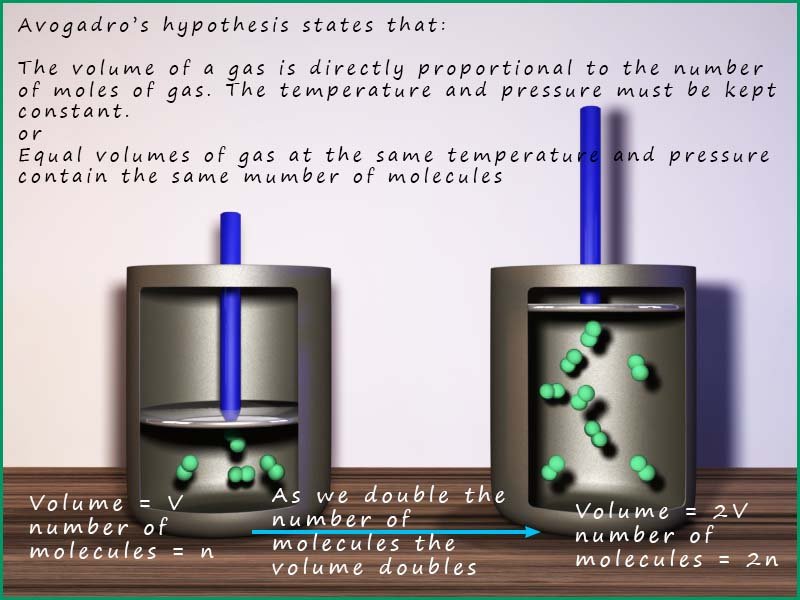
In 1811 Amedeo Avogadro proposed what is now called Avogadro's Hypothesis.
He proposed that: Equal volumes
of gases at the same temperatur and
pressure contain equal numbers of particles.
At 273k or 00C
and 101kPa for example 1 mole of any gas will occupy a volume
of 22.4 litres or 22.4 dm3.
These conditions of temperature and pressure
are called standard temperature and pressure (S.T.P). At 298K
(250C) and 101kPa 1 mole of any gas will occupy 24 litres or
24 dm3; these conditions are
called room temperature and pressure.