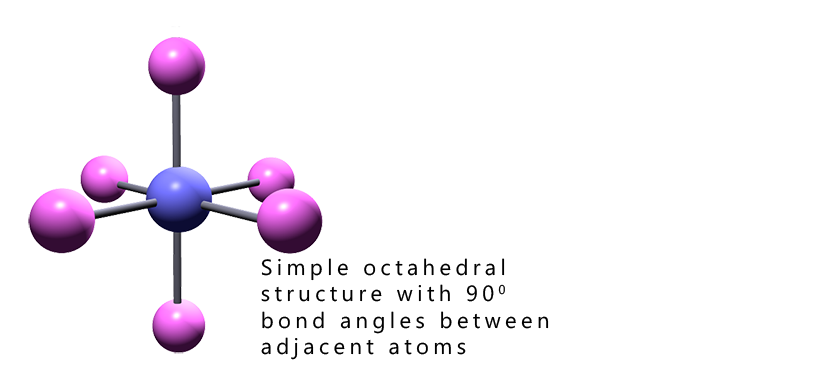
This page follows from on the pages on from those on finding lone pairs in trigonal bipyramidal molecules and you should be familiar with VSEPR theory and how to work out the basic shapes of molecules to get the most from this page which deals with working out the shapes of octahedral based molecules.
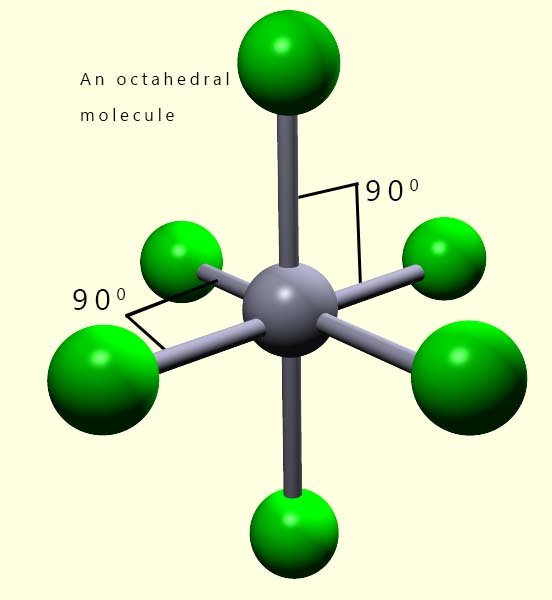
Working out the shape of octahedral molecules with lone pairs of electrons is more straight- forward than that with trigonal bipyramidal molecules. The reason for this should be fairly clear; all the positions in an octahedral molecule are equivalent, the bond angles between all adjacent atoms in octahedral molecules are 90°. Whereas in trigonal bipyramidal molecules we had to consider two different locations, that is the axial and equatorial positions; however this is not the case with octahedral shaped molecules. In an octahedral all positions are equivalent.
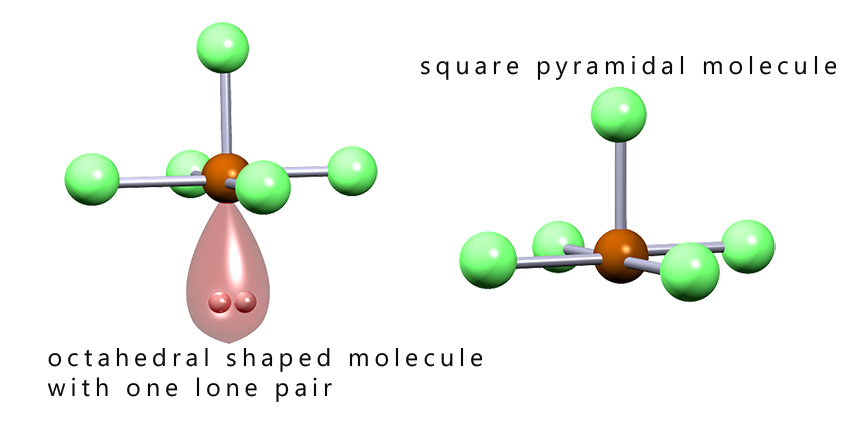
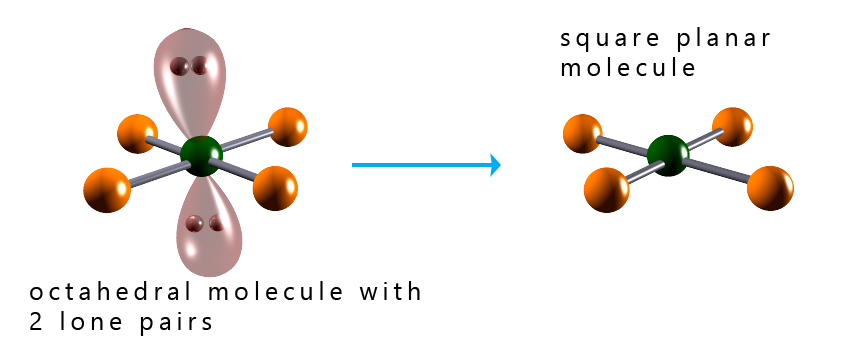
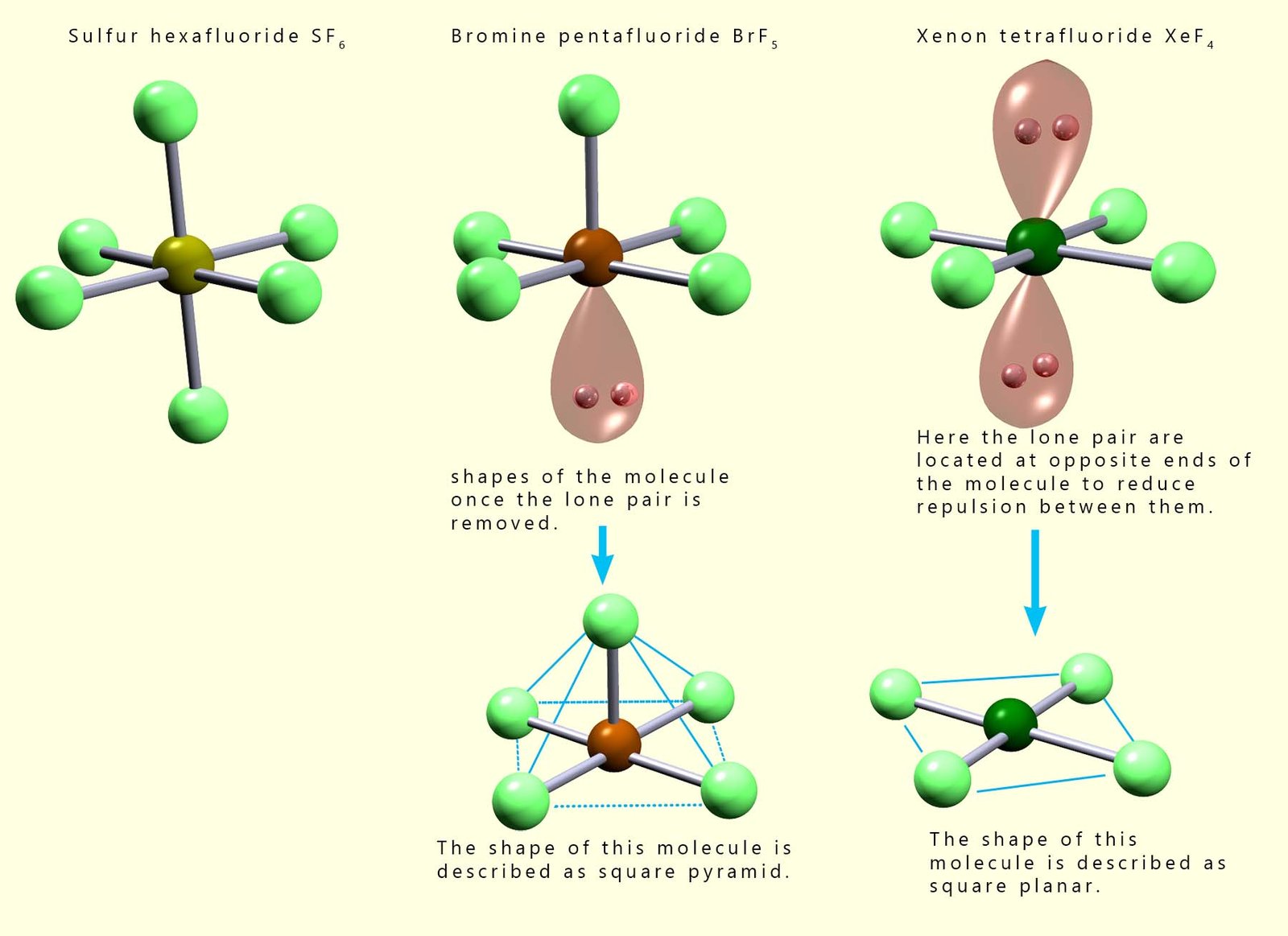
The image below shows the shapes of several octahedral molecules that contain lone pairs of electrons. Recall that we do not consider the presence of lone pairs of electrons when determining the final overall shape of a molecule, but it is important to identify the presence of any lone pairs as these can affect the bond angles present in a molecule. From the image below we can say that:
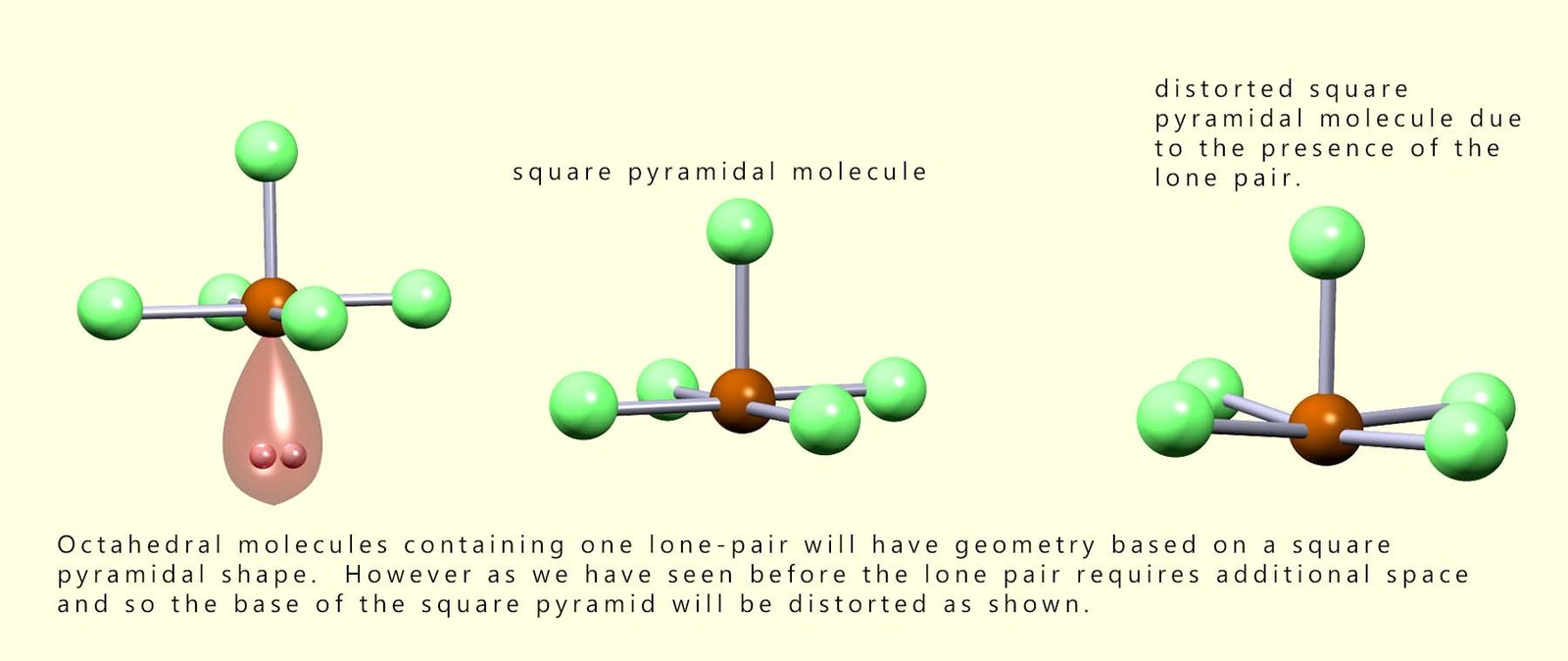
As we have seen with trigonal pyramidal molecules the presence of lone pairs of electrons will have an effect on the bond angles present in molecules. The lone pair present in a square pyramidal molecule will push the bonding pairs of electrons up and so distort the base of the square pyramid, this is outlined in the diagram below:
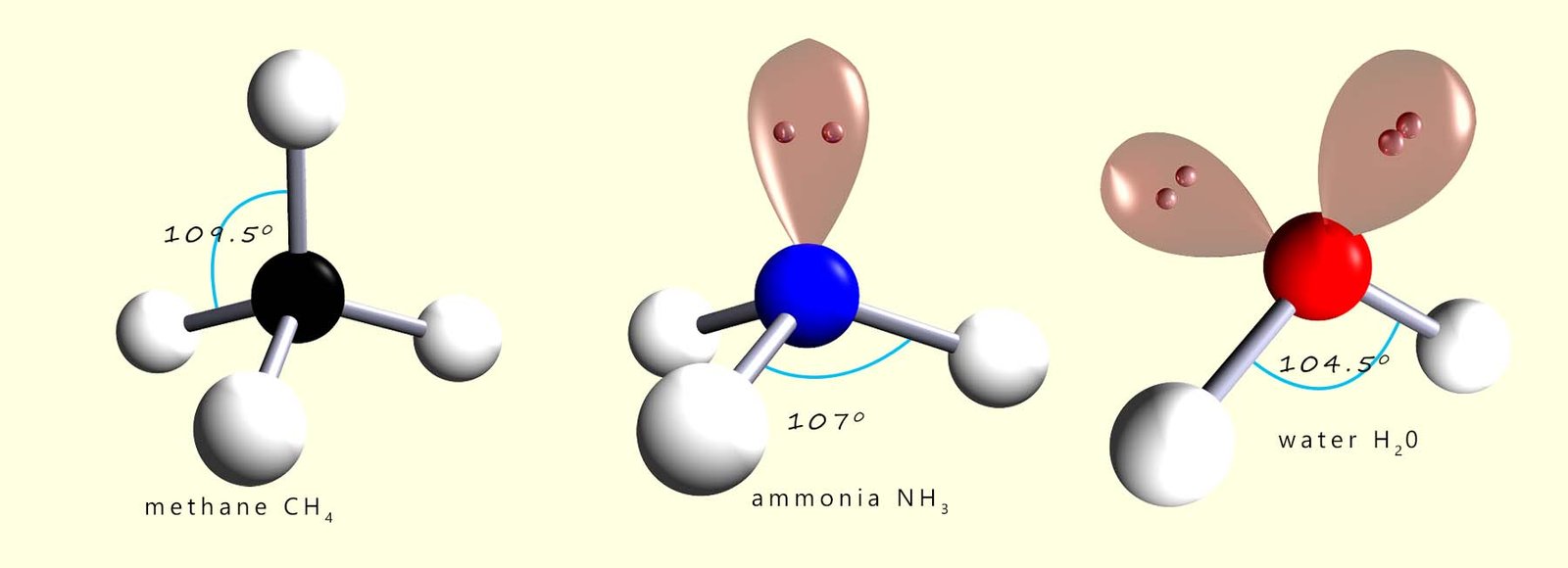
The 3 molecules shown below all have shapes based on a tetrahedral arrangement around the central atom, however as we have seen that lone pairs of electrons require more space than bonding electron pairs and this results in the reduction of the bond angles between the bonding pairs as shown below.
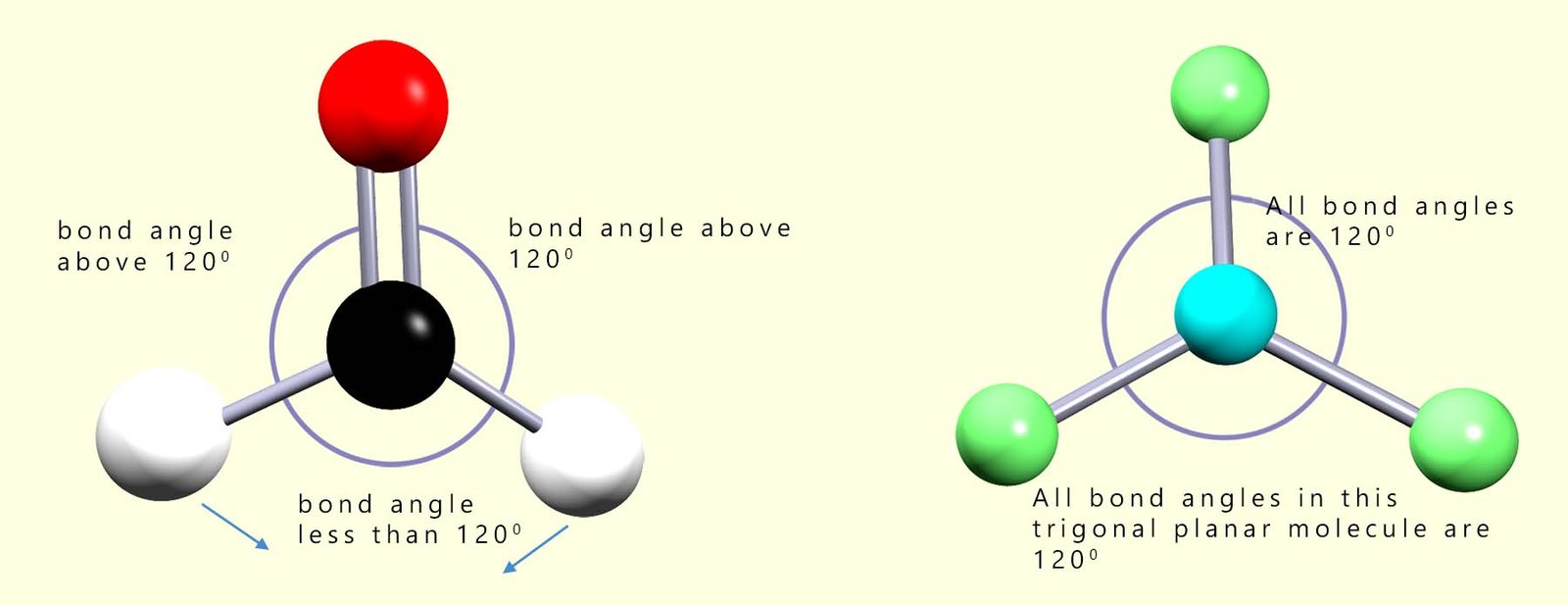
Molecules with multiple bonds; that is double or triple covalent bonds between the atoms show a similar effect. Double bonds for example contain a higher electron density than single covalent bonds and so will repel other bonding electron pairs in a manner similar to that of lone pairs. In the image shown below there are 2 molecules which have a trigonal planar structure. In a trigonal planar molecule we might expect bond angles of 120°; however as shown below you can see that the double bond requires more space than the single bonds in trigonal planar molecules and the bond angles will be not be the expected 120° but instead will be reduced.
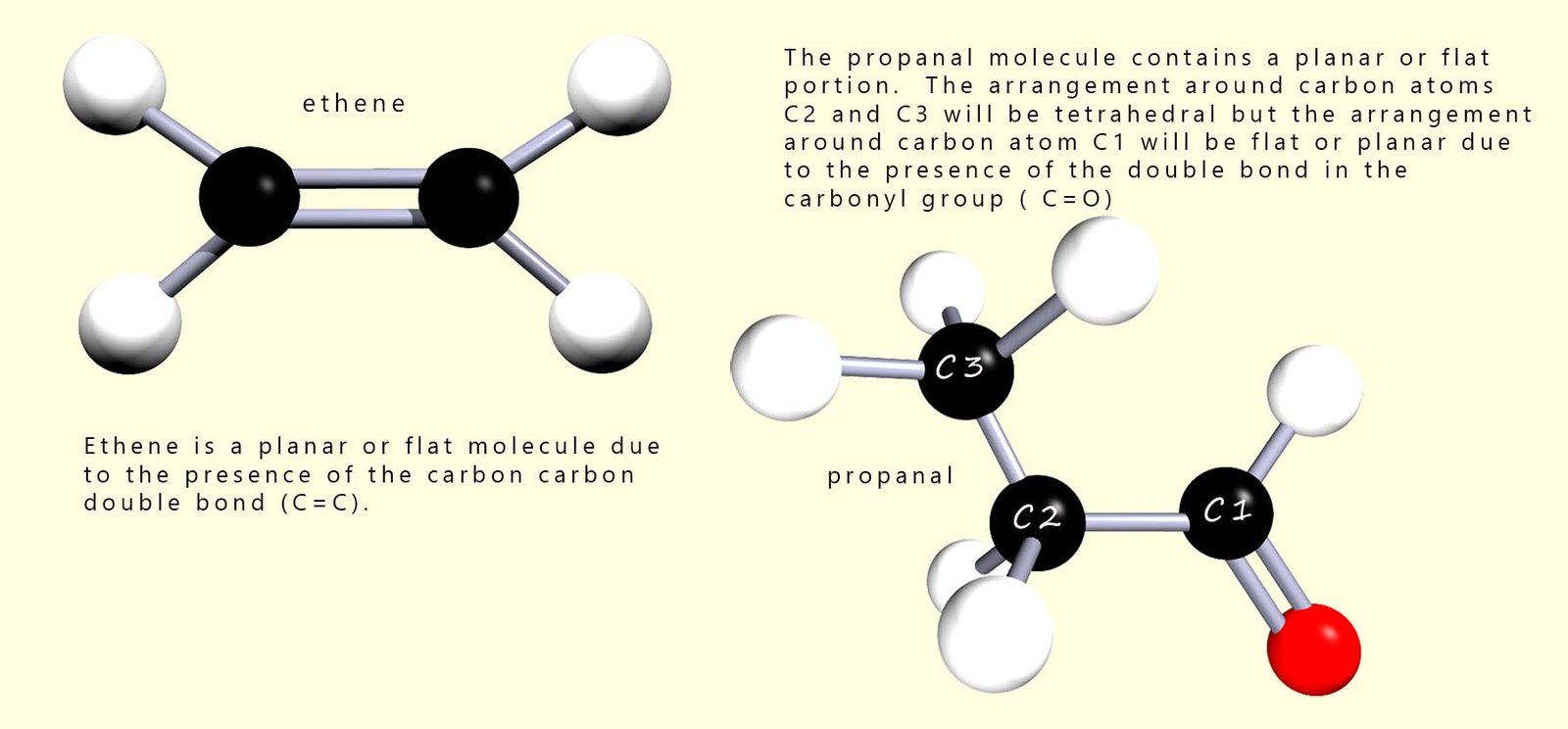
Molecules which contain double or triple bonds will be planar or flat around the site of these multiple bonds. This is a consequence of the atomic orbitals that overlap in forming these multiple bonds. Alkenes for example will be flat or planar molecules around the C=C bond as will aldehydes and ketones around the site of the carbonyl group (C=O). This is shown in the image below:
The activity below will quickly review the shapes of octahedral based molecules with 0, 1 and 2 lone pairs present; simply select the number of lone pairs present using the buttons below: