

A crystalline substance is one in which the atoms or ions that make up the structure are arranged in a very ordered and uniform way. A structure where the atoms or ions are arranged in a random or disordered way is called a non-crystalline or amorphous structure. As a rather simple example we could say a brick wall is crystalline; the brick are laid in a very ordered and regular way but if you knock the wall down then you end up with a large pile of brick; this would be a disordered or amorphous arrangement.
Carbon is perhaps one of the most remarkable elements in the periodic table; it forms such a wide
variety of substances which can range from small to medium sized molecules
to others which consist
of giant covalent network solids such as diamond
and graphite.
The structures of these allotropes of
carbon are shown below. You can clearly see that the structures of both diamond
and graphite are very
ordered, that is they are both crystalline
in nature. However they have very different properties due to
the way in which the carbon atoms are arranged and bonded together in each structure.
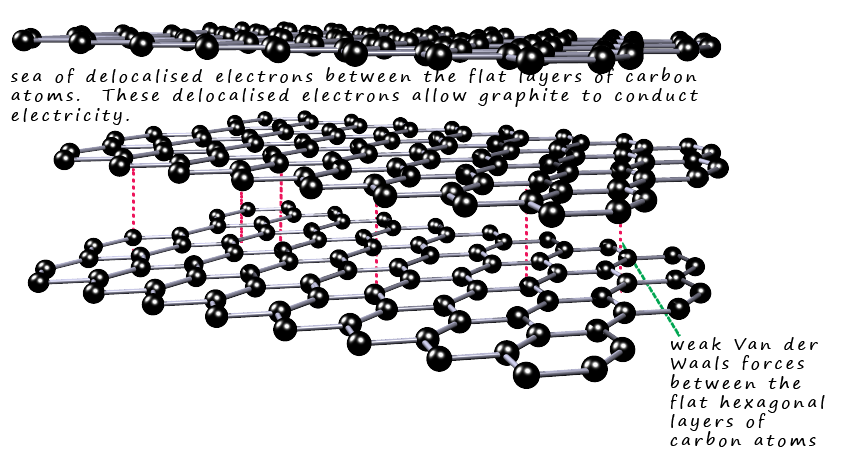
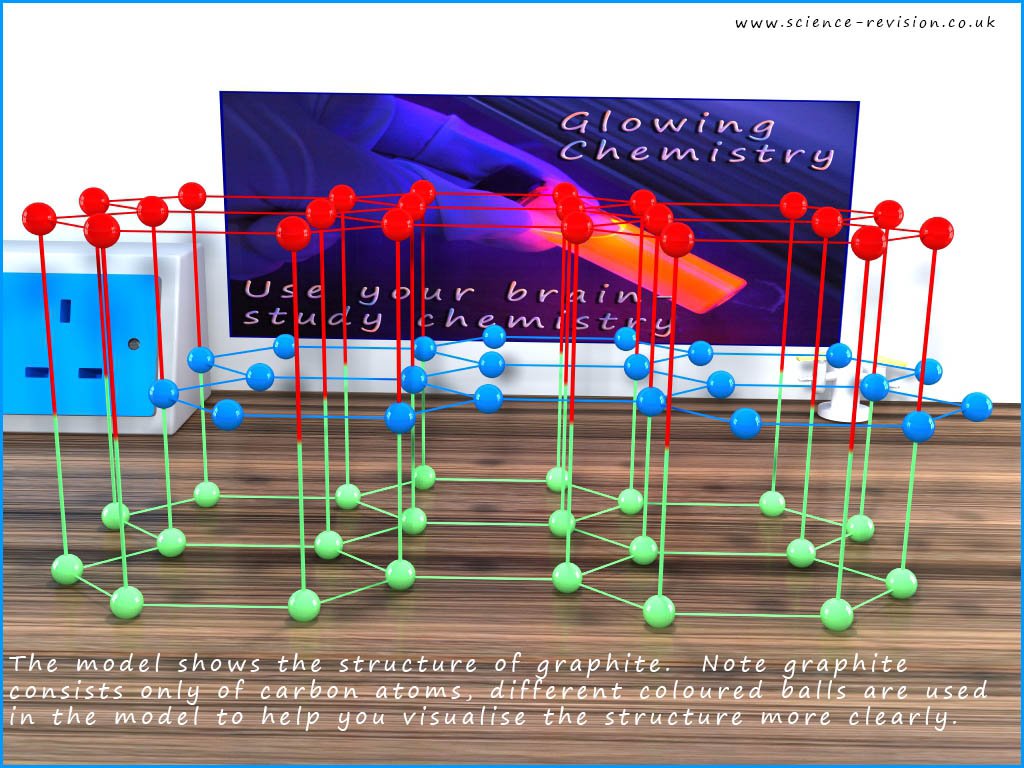
Between the flat sheets of carbon atoms are weak Van der Waals intermolecular bonds. These are weak bonds and are easily broken. One of the main uses of graphite is as an industrial lubricant. Pencil leads are made of graphite and if you rub a pencil over a sheet of paper for a few second to leave a thin layer of graphite on the paper then rub your finger over the graphite layer you will feel just how slippery graphite is. You can perhaps imagine that when you rub the graphite pencil lead on the paper the weak intermolecular Van der Waals bond holding the hexagonal layers together break when you push the pencil along, this will leave the flat hexagonal layer intact on the paper. The bonds in this layer of hexagons will not break easily since they are strong covalent bonds. The image below shows the layered structure of graphite:
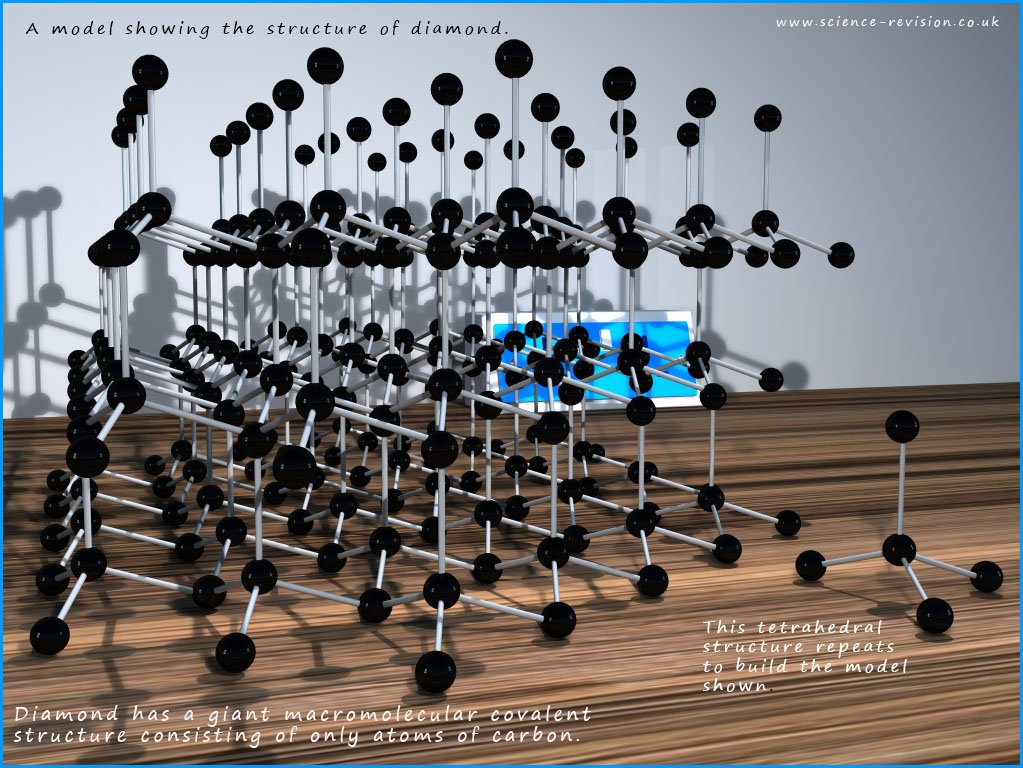
Diamond like graphite
consists of only carbon atoms; but whereas graphite is a soft,
flaky material that conducts electricity diamond is the hardest
known natural substance and it is also
an electrical insulator. Though like graphite
when heated to a very high temperature it tends to
sublime before it melts.
The reason why diamond has different properties from graphite is due to
the bonding within the structure.
Diamond has an ordered crystalline structure
like graphite but
that is where the similarities end. Each carbon atom in diamond makes 4 strong covalent bonds.
All the electrons in the carbon atoms valence shell are involved in
forming covalent bonds. They
are held in these bonds and so are not free to move as they are in graphite,
this explains why
diamond is an electrical insulator.
In the image below you can see that each carbon atom forms 4
covalent bonds which are all the same length, unlike in graphite where the bond lengths
between the
layers are much larger than the bonding within the layers. In diamond
each carbon atom forms a tetrahedral
arrangement; with bond angles of 109.5 with its neighbouring atoms.
At first glance the structure of diamond looks impossible to draw, however it is fairly easy to draw a simple outline of the basic structure. Remember each carbon atom is in a tetrahedral arrangement but also the whole structure is built up of six membered rings of carbon atoms fused or stuck together. Graphite also consists of 6 membered rings of carbon atoms, but in graphite the rings are flat whereas in diamond they are puckered or bent into a chair like structure. No matter from which direction you view the diamond structure from you always see the same 6 membered puckered rings of carbon atoms - that is the structure is highly symmetrical and looks the same from all angles. The diagram below shows you how to draw a basic outline of these fused or puckered rings that build up the diamond structure, the final drawing shows the repeat unit which is used to build up the diamond structure.

If you study the large 3d model above of diamond you should be able to see lots of the puckered 6-membered rings of carbon atoms that repeat and build up the model shown in the image above.

Unlike diamond and graphite these newly discovered fullerenes have a molecular structure and not the giant macromolecular structures found in diamond and graphite. The carbon atoms in these football like structures is similar in some ways to that found in graphite. The carbon atoms make 3 covalent bonds and have 1 spare electron which is delocalised over the molecule. However unlike in graphite the delocalised electrons are trapped within the molecule and so these buckyballs are electrical insulators. Buckyballs being molecular will dissolve in some organic solvents; this is not the case with giant macromolecular solids.