

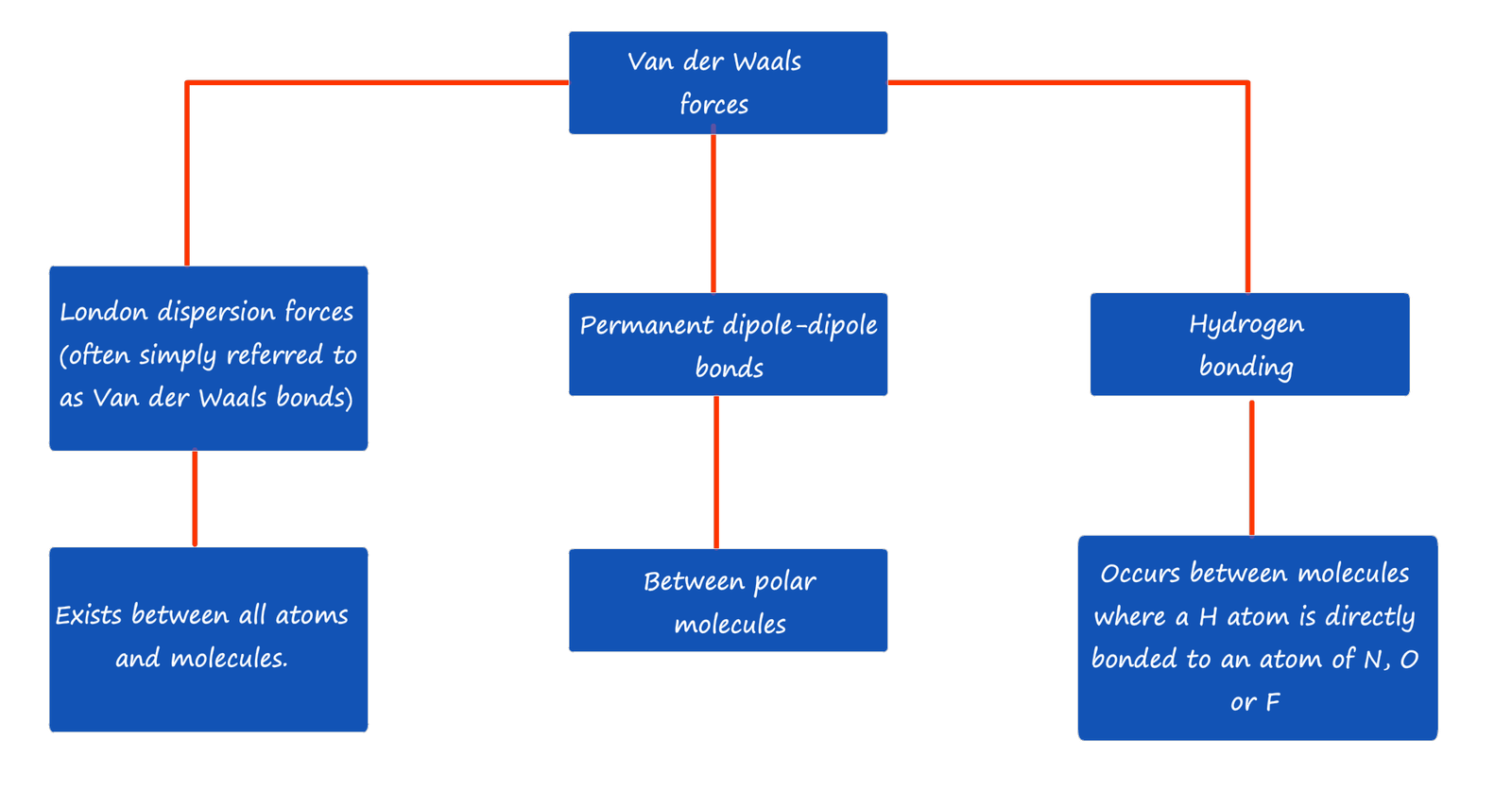
 However some exam boards, AQA for example do not discuss London dispersion forces but simply use the term Van der Waals force instead. So don't get confused by this, London dispersion forces are often simply described as Van der Waals forces by some exam specifications! Just check the exam board specification to ensure you are using the terminology they want. Now this page discusses London dispersion forces or Van der Waals force, the name may vary depending on the exam board but the type of interaction between atoms and molecules is the same. This type of intermolecular bond arises due to temporary fluctuations in electron distribution within a molecule or atom. So let's go!!
However some exam boards, AQA for example do not discuss London dispersion forces but simply use the term Van der Waals force instead. So don't get confused by this, London dispersion forces are often simply described as Van der Waals forces by some exam specifications! Just check the exam board specification to ensure you are using the terminology they want. Now this page discusses London dispersion forces or Van der Waals force, the name may vary depending on the exam board but the type of interaction between atoms and molecules is the same. This type of intermolecular bond arises due to temporary fluctuations in electron distribution within a molecule or atom. So let's go!!
Consider the atoms in a noble gas such as radon for a second. In your year 7 science lessons you might have discussed the fact that the forces of attraction between solid particles are greater than the forces of attraction between liquid particles which are greater than those between gas particles; but what are these forces that exist between atoms and molecules? If we cool down a noble gas such as radon it liquefies, that is the radon atoms are able to stick together to form a liquid which as we have just said has fairly strong forces of attraction between the particles present in the liquid, but what are these forces exactly? The radon atoms are electrical neutral, non-polar atoms. We know that noble gases don't react easily since they have full electron shells and they consist of individual atoms but in order to liquefy radon or even to solidify it there must be forces of attraction present between these individual atoms.
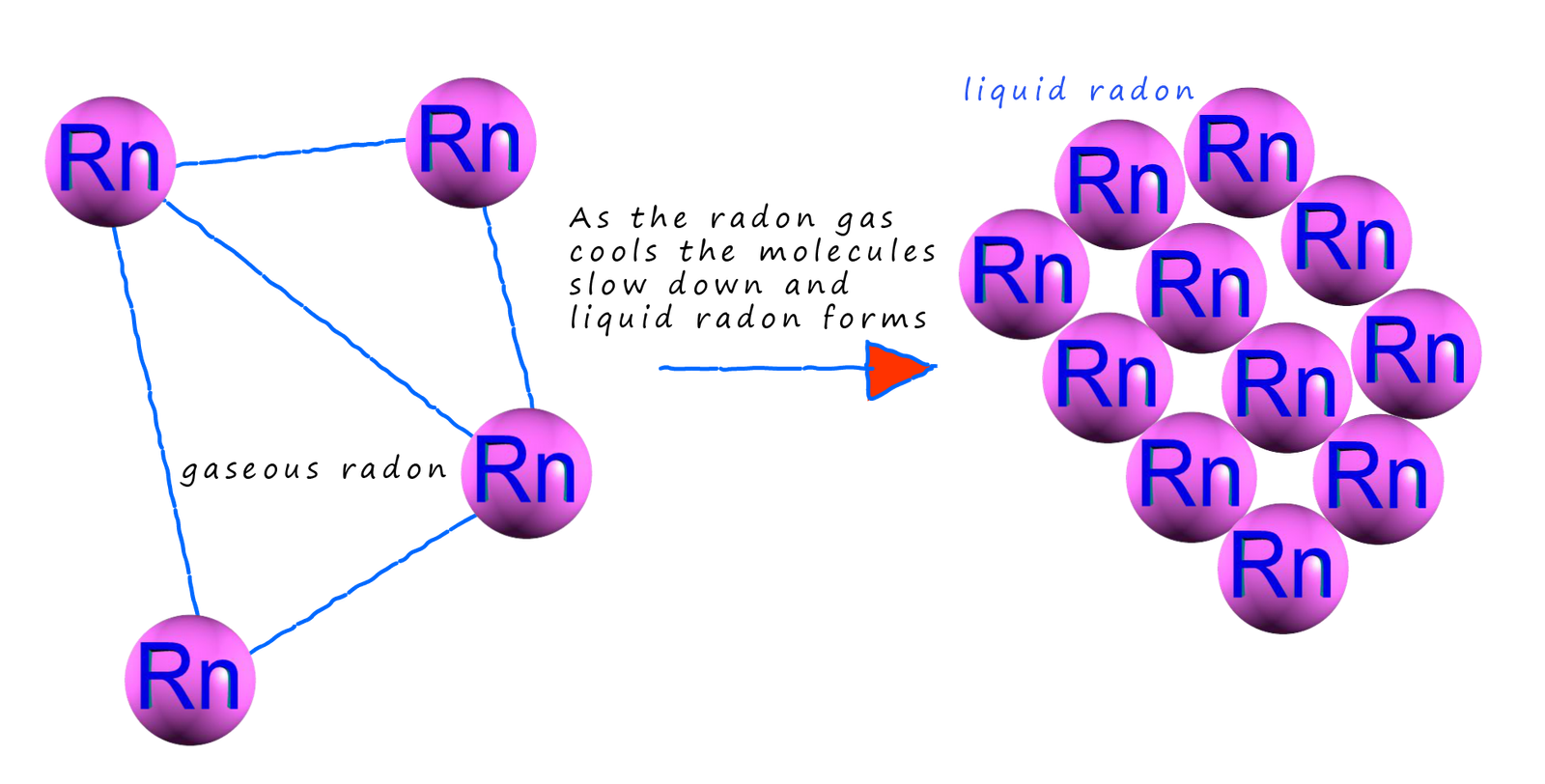
The electrons within an atom or a molecule are in constant motion; this means that the distribution of negative charge within the atom or molecule is going to be random and asymmetrical at times. This uneven distribution of electrons within an atom or a molecule creates of partial negative charge (δ-) in areas where the density of the electrons is high and other areas of partial positive charge (δ+) where the electron density is lower. This random movement of electrons will therefore create dipoles within the atoms/molecules. However these dipoles will only be temporary since the electrons are in constant motion. However they will last long enough to influence the electron distribution in any neighbouring atoms/molecules they come close to. This will induced temporary dipoles in these neighbouring atoms/molecules. This is shown below using helium atoms as an example.

You can see that:
However it is not only the electrons that are in constant motion; the atoms themselves in the gas phase move at high speed in a random manner. Even though there are large spaces between the atoms as they cool they get closer to each other and this can also lead to the formation of temporary induced dipoles within the atoms or molecules. As shown below:

These temporary induced dipoles have a sort of chain reaction affect in that they generate dipoles in neighbouring
atoms; the attraction of one atom/molecule to the oppositely charged end of another molecule is called
a Van der
Waals force or London dispersion force.
The size of these Van der Waals forces increases as the number of
electrons in the atom/molecule increases, it
also increases with molecular size.
In the diagram below the red and yellowish ovals surroundings the molecules represent the skin of electron density
which
we can imagine as covering the molecules. Due to uneven electron distribution within the molecules at any
one time dipoles are temporarily generated, the magnitude and number of intermolecular Van der Waals forces
increases with
increasing number of electrons and increasing surface are of the molecules. There is much more Van der
Waals bonding present in pentane than in the much smaller ethane molecules as shown below simply because pentane is a larger molecule with more electrons.

It is not only the size and number of electrons
present that will influence the amount of Van der Waals bonding/dispersion forces present, the
shape
of the molecules is also an important consideration. Now Van der Waals/dispersion forces is/are
a form of intermolecular bonding that relies on
the molecules/atoms being able to get close enough to each other to influence the electron distribution. As an
example of how important shape is
consider pentane, a hydrocarbon molecule with the formula C5H12. The image below shows
2 isomers of
pentane. The straight chain isomer will be able to get much closer to another neighbouring molecule than will the branched isomer. This means that
there will be much more Van der Waals/dispersion forces present in the straight chain isomer of pentane. This means that the straight chain isomer will
have slightly differ physical properties, for example it will have a higher boiling point and viscosity than the branched isomer due to the presence of
additional intermolecular Van der Waals/dispersion forces.
Both the molecules shown below have the formula C5H12. Since both molecules have the same number of atoms of carbon and hydrogen present there will be the same number of electrons present within each molecule. However both molecules clearly differ in their shape
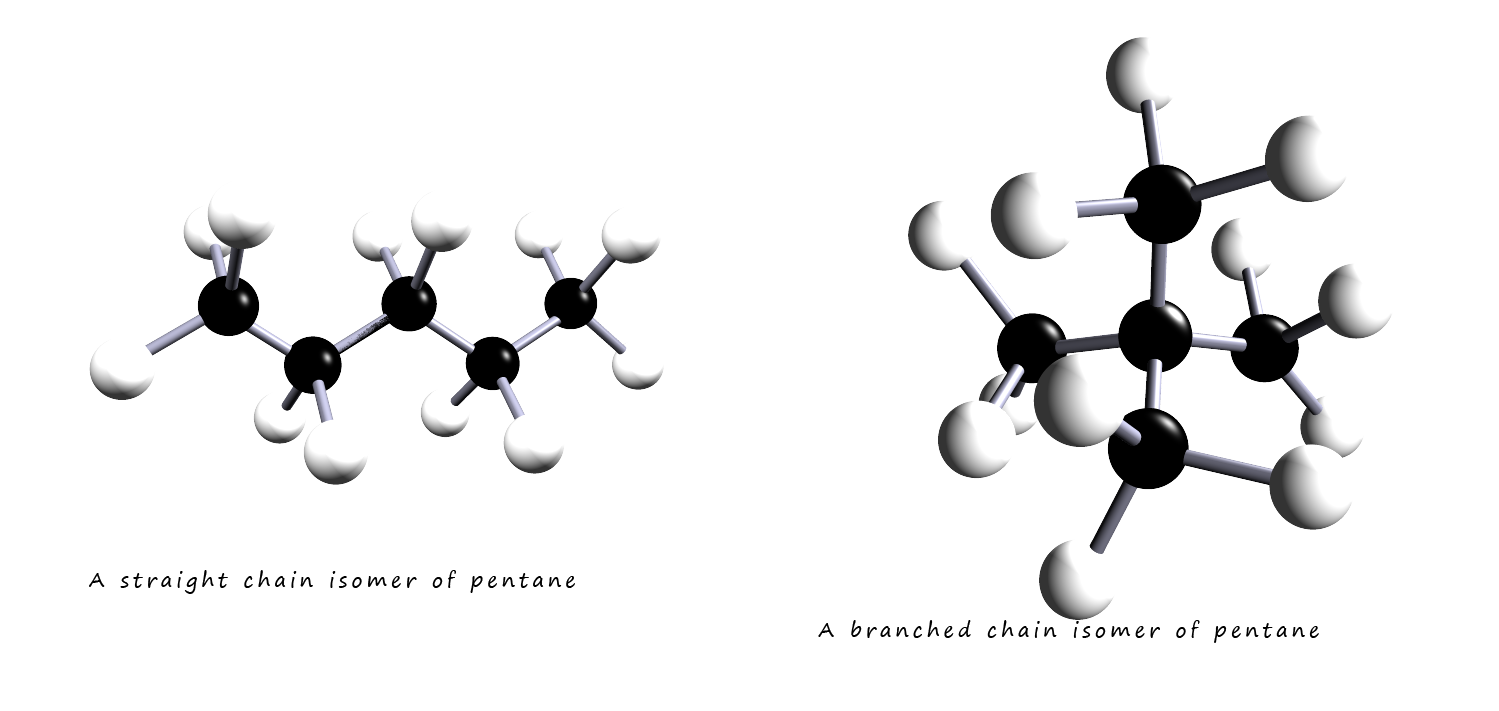
Van der Waals/dispersion forces act between neighbouring molecules and rely on the neighbouring molecules to be able to get close enough and be able alter and change the electron distribution in each molecule. This means molecules which have larger surface areas will be able to form more Van der Waals/dispersion intermolecular bonds than molecules which due to their shape only have limited areas of contact.
Finally it is worth noting that Van der Waals forces/dispersion forces act between all atoms and molecules and they are in addition to any other intermolecular forces or bonds that are also acting. However you should bear in mind that Van der Waals bonding is very weak in comparison to intramolecular bonding such as ionic or covalent bonding.
