
 The bonding which holds giant structures such as diamond, graphite and ionic lattices as well as small molecular substances together will be either covalent, ionic or metallic bonds in the case of metals. These different types of bonding are all
very strong, now this type of bonding is often called the intramolecular bonding. That is the bonding "within" the molecule or giant structure.
The bonding which holds giant structures such as diamond, graphite and ionic lattices as well as small molecular substances together will be either covalent, ionic or metallic bonds in the case of metals. These different types of bonding are all
very strong, now this type of bonding is often called the intramolecular bonding. That is the bonding "within" the molecule or giant structure.
However there is another type of bonding which acts between different covalent molecules and individual atoms such as those found in the noble gases. This type of bonding is called intermolecular bonding and it is very weak in comparison to covalent, ionic or metallic bonding, that is the intramolecular bonding.
In gcse chemistry we simply learned about the existence of this intermolecular bonding between different molecules and atoms and the affect it had on some of the physical properties of small molecules such as their melting, boiling points viscosity and solubility.
In A-level chemistry you will need to become more familiar with the different types of intermolecular bonding and how each type acts and the various effects it has on many of the properties of atoms and molecules.
The forces of attraction or intermolecular bonding between different covalent molecules and individual atoms such as those found in the noble gases are often referred to as Van der Waals forces or Van der Waals bonding. There are three main types of intermolecular bonding or Van Der Waals bonding that you need to become familiar with; these are:
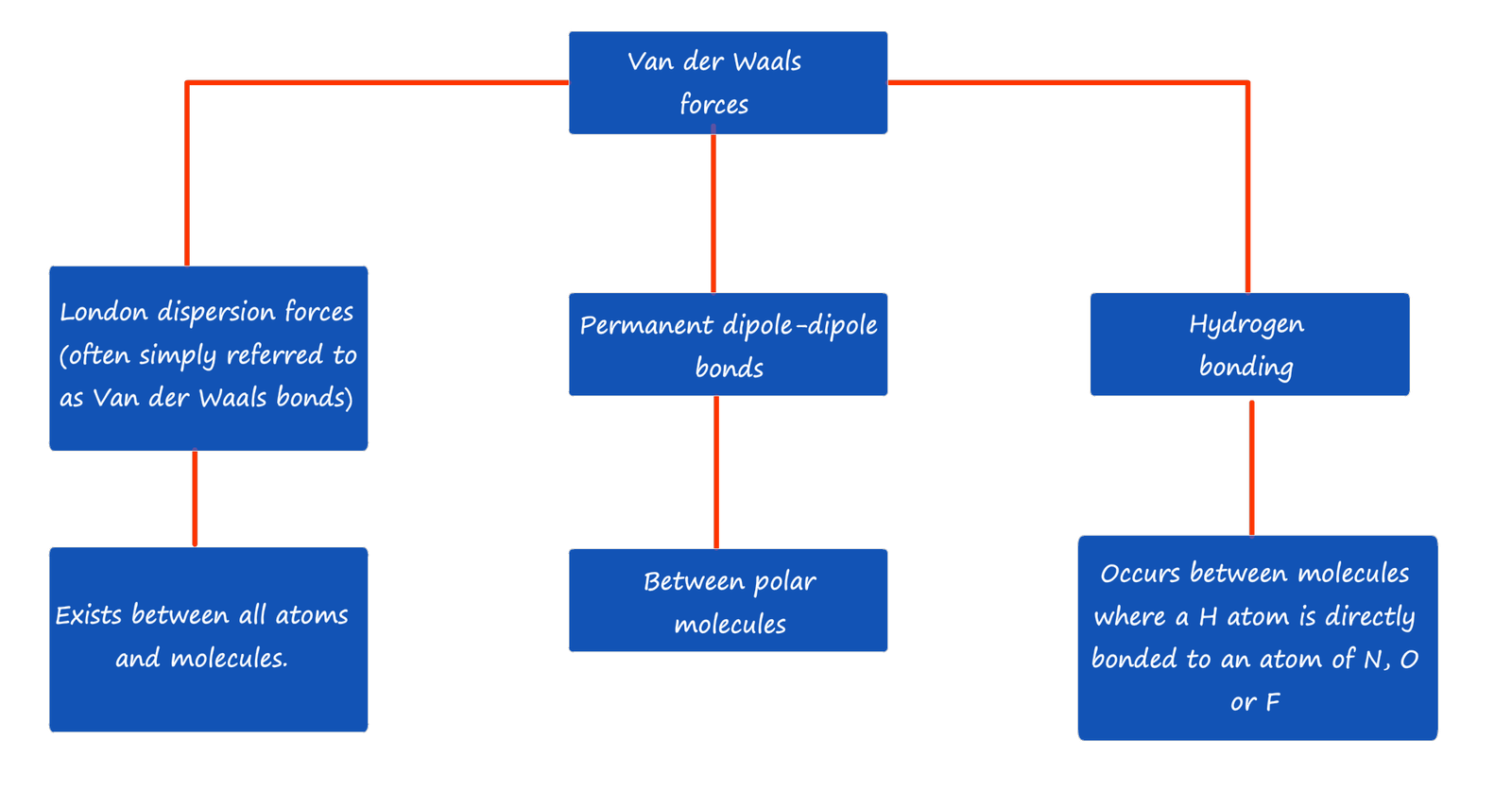 Note: Some exam specifications, AQA for example do not use the term London dispersion forces or dispersion forces but simply refer to these as Van der Waals bonds or forces. Simply check your exam specification or textbook to ensure you use the correct term. For more information on each of these types of intermolecular bonds simply click the links at the top or bottom of the page.
Note: Some exam specifications, AQA for example do not use the term London dispersion forces or dispersion forces but simply refer to these as Van der Waals bonds or forces. Simply check your exam specification or textbook to ensure you use the correct term. For more information on each of these types of intermolecular bonds simply click the links at the top or bottom of the page.