
Hydrogen bonding is a type of dipole-dipole attraction between
molecules containing O-H, N-H and H-F groups. It is also
the strongest form of intermolecular bonding with hydrogen bonds in water having around 5% the bond strength of the
O-H covalent bonds in water. Hydrogen bonding
is found in covalently bonded
molecules which contain a hydrogen atom bonded to
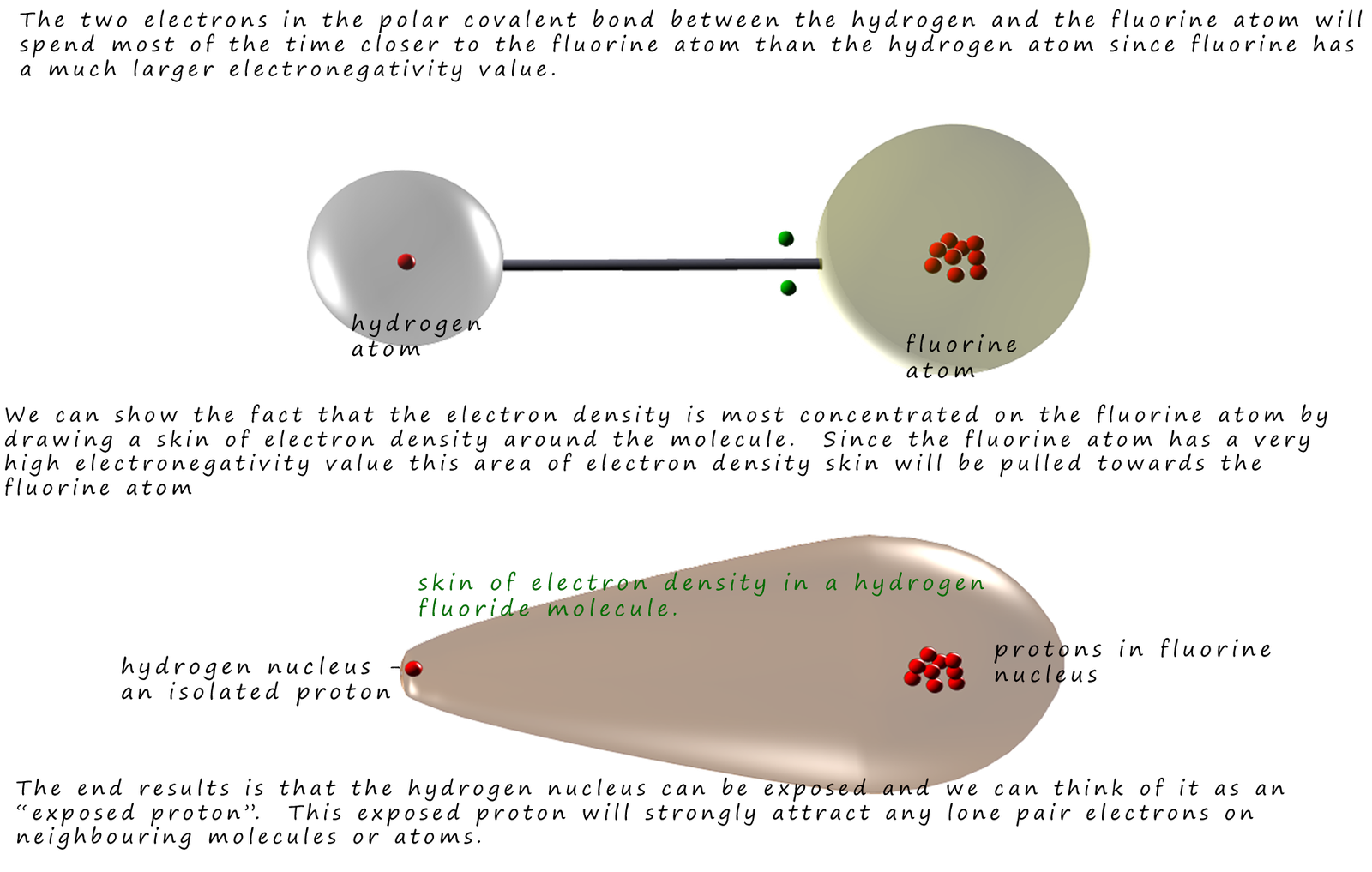
nitrogen, oxygen and fluorine atoms only. The
reason for this is that N, O and F are the three most electronegative elements in the periodic table. This means that
the electron density in the polar covalent bond between the hydrogen atom and any N, O or F atoms
attached to it will be very much concentrated towards the electronegative element and away from the
hydrogen atom. This will leave an exposed hydrogen nucleus or proton
with a large δ+ charge which
can form a very strong intermolecular bond with the lone pairs of electrons on
the small atoms of nitrogen, oxygen
and fluorine. This is shown in the diagram below which uses the hydrogen fluoride molecule as an example:
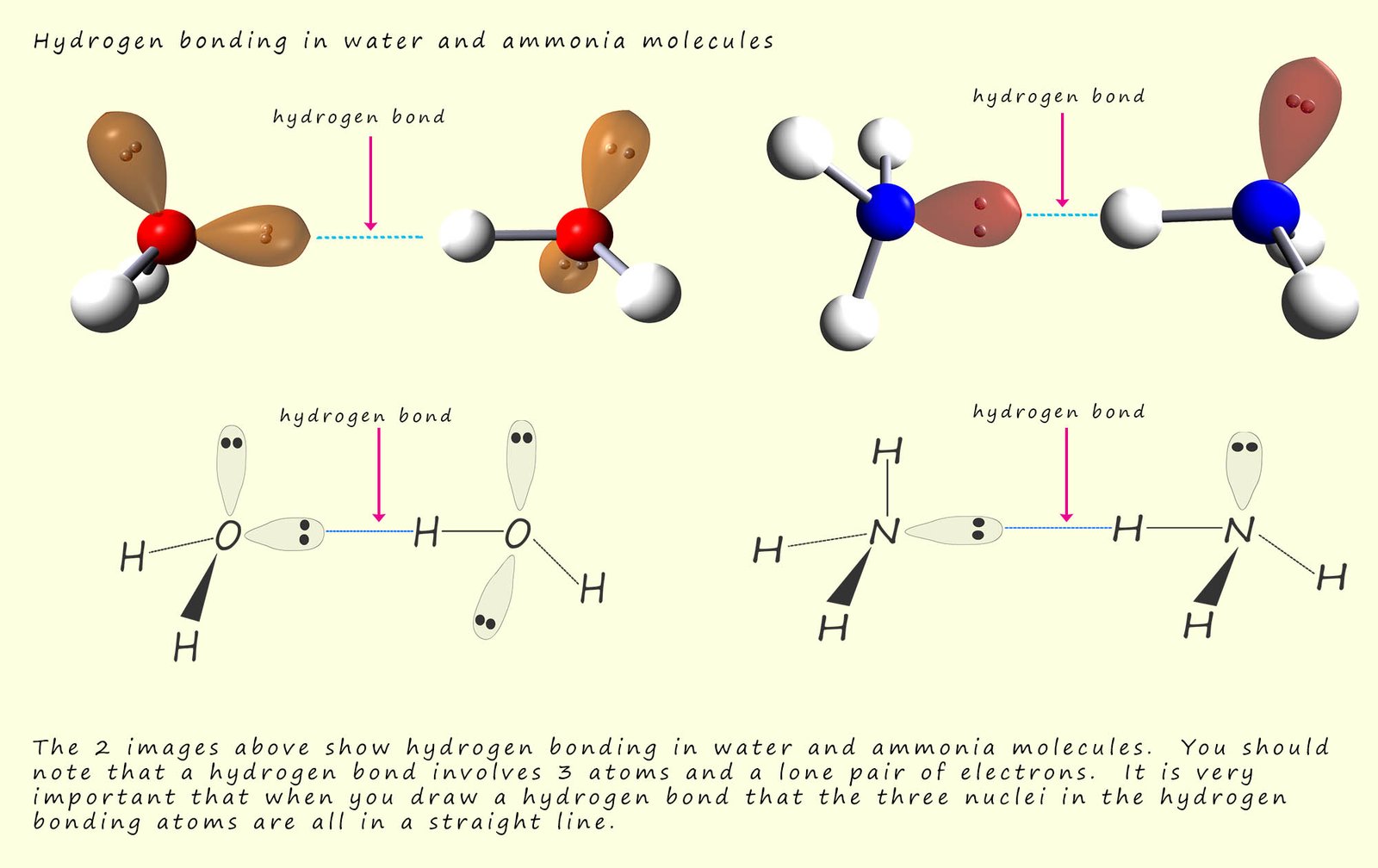
For a hydrogen bond to form you need 3 atoms and a lone pair of electrons, it is important that when you are asked to draw a hydrogen bond that the atoms and the lone pair of electrons involved in the hydrogen bond are in a straight line with each other. The diagram below shows how one hydrogen bond can form in molecules of water and ammonia. If you look at the hydrogen bond in the water molecule you will see that the oxygen atom-----hydrogen atom---lone pair ----oxygen atom involved in forming the hydrogen bond are all in a straight line. It is a similar story with the hydrogen bond in the ammonia molecule; the three atoms involved in forming the hydrogen bond MUST be in a straight line.
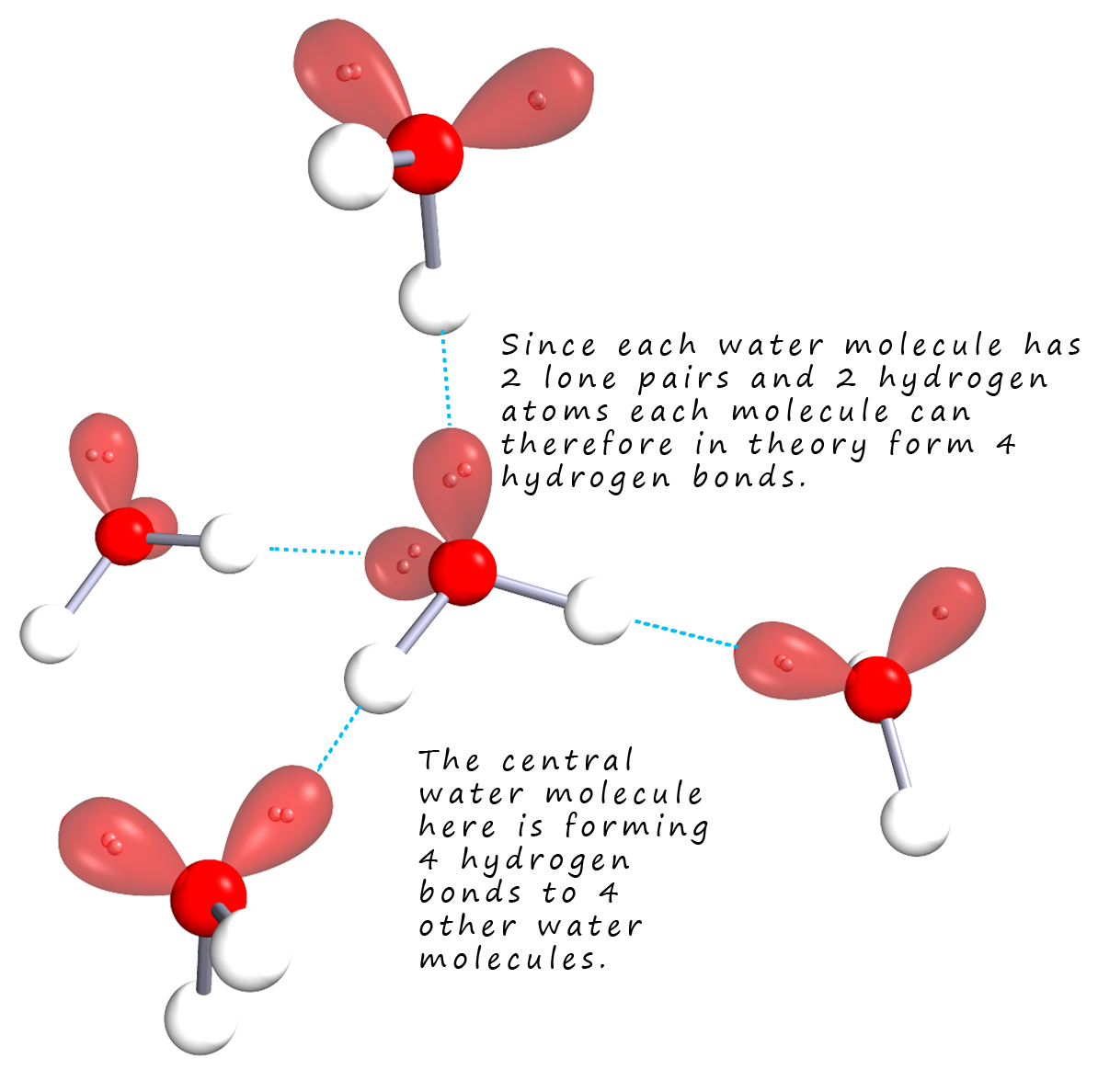
The hydroxyl group (-OH) is very common in many molecules such as water, carboxylic acids and alcohols. Any molecule which contains this functional group can undergo hydrogen bonding. Hydrogen bonding is particularly strong in water since each water molecule is able to form 4 hydrogen bonds as shown in the image below (note the number of hydrogen bonds formed by each molecule will vary with temperature and the kinetic energy of the particular water molecules). Although molecules such as hydrogen fluoride and ammonia can in theory form just as many hydrogen bonds as water in reality they rarely do. This extended hydrogen bonding found in water is responsible for many of its characteristic properties including a very high melting and boiling points and viscosity considering its very small molecular size and mass it is also responsible for the very high surface tension found in water.
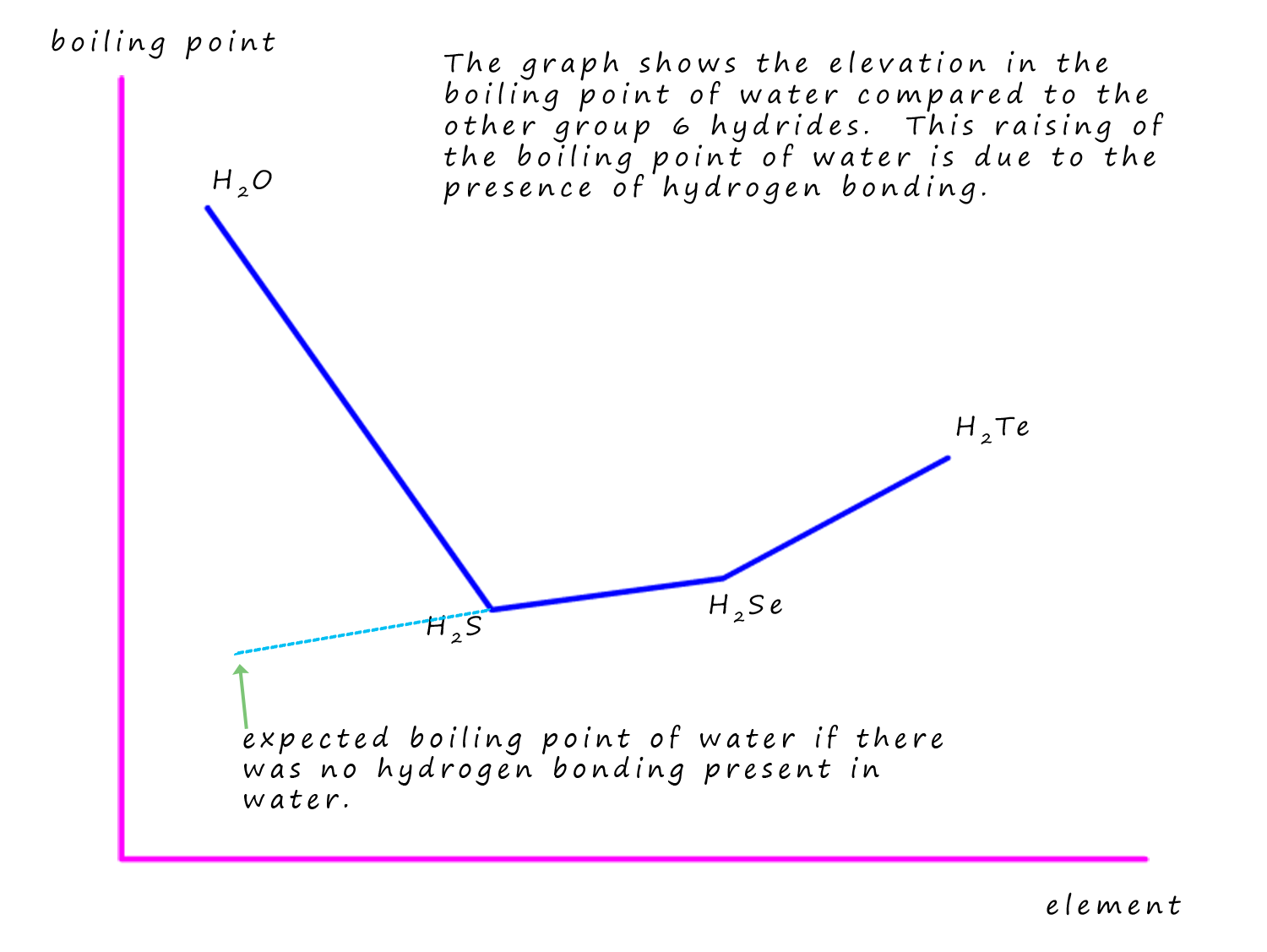
The presence of hydrogen bonding can help explain many of the unusual changes in the physical properties of many of the compounds which contain hydrogen bonding. As an example consider the hydrides of the group 6 elements: O, S, Se and Te. We would expect the boiling points of the hydrides to increase down any group simply due to the increase in the mass of the molecules and the amount of Van der Waals bonding (London dispersion forces) present between neighbouring molecules. So in this case hydrogen oxide (water- H2O) should have the lowest boiling point and tellurium hydride (H2Te) to have the highest boiling point. However this is not what is observed; in fact the smallest molecule has the highest boiling point; the total opposite of what we might have expected! Water has a much higher boiling point than would be predicted; this is shown in the graph below. This hugely inflated boiling point for water tells us that much more energy is needed to separate the water molecules than would be expected. This additional energy is due to the presence of hydrogen bonding in water molecules whereas only weak Van der Waals (London dispersion forces) intermolecular bonding is found in the other group 6 hydrides.
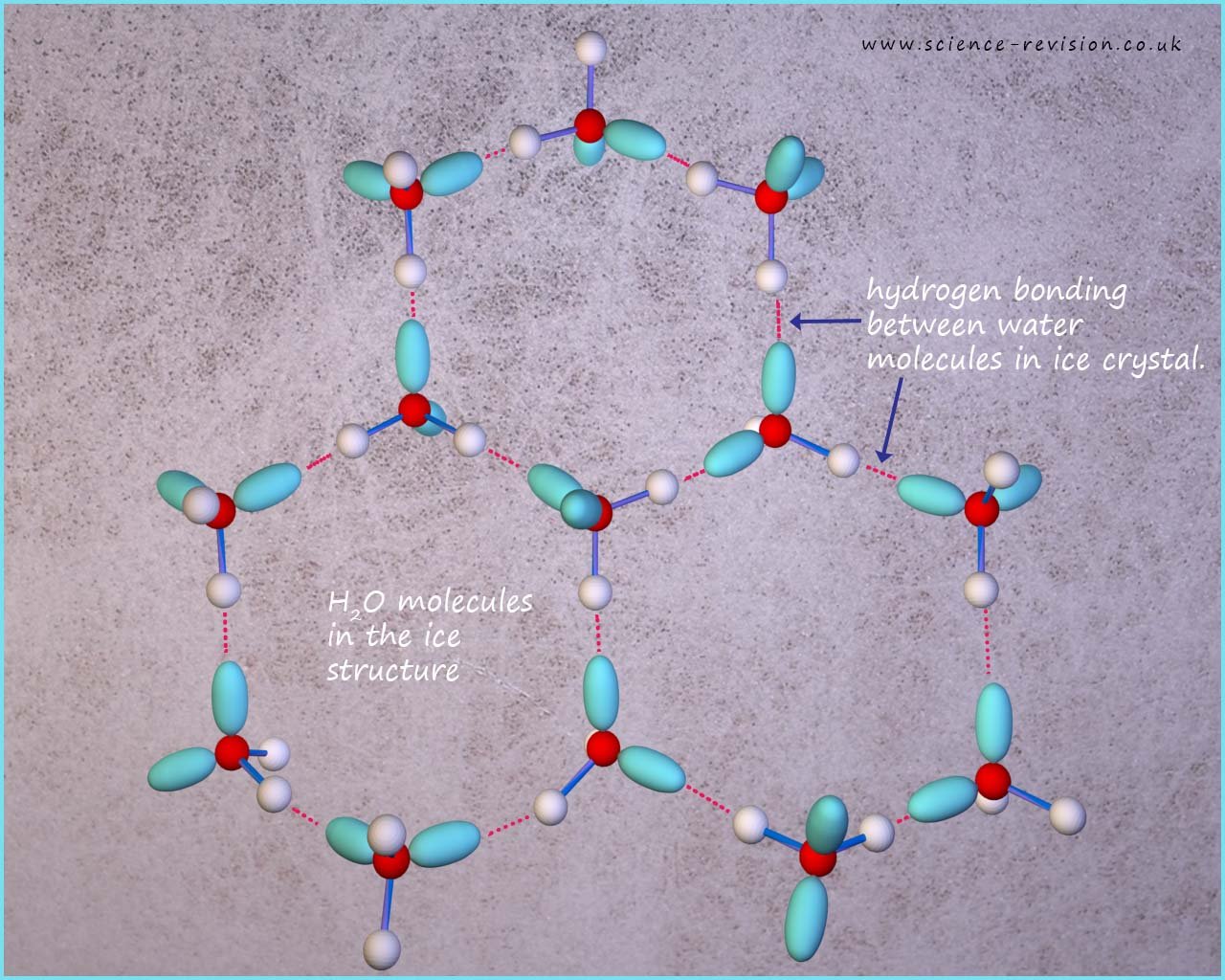
A water molecule has two lone pairs of electrons; this will enable each molecule of water to form 4 hydrogen bonds as discussed above. The image below shows how the water molecules are arranged in ice.
 When water is in the liquid state the water molecules are free to move and slide over each other, this means
that the hydrogen bonds must be continually breaking and
reforming as each water molecule moves. However
as the temperature drops close to the freezing point of water the movement of the
water molecules slows and
at 40C the density of the water reaches it maximum, then below 40C the water molecules
start to
move apart as more and more hydrogen bonds start to form. The problem is that these hydrogen bonds prefer specific angles. To maximize these angles in the ice lattice structure, the water molecules end up spaced farther apart than they would be in liquid water. This creates the open, airy structure of ice and the water reaches its
freezing point the water molecules will be unable to move because they are held in place
by the hydrogen bonding between adjacent molecules.
In order to form the ice structure the water molecules
are not as densely packed as they are in water, this explains why ice is less dense than water and floats on it.
It is easy to see when you look at the ice structure how open it is. This is a very unusual property since for
most substances the
solid state is denser than the liquid state.
When water is in the liquid state the water molecules are free to move and slide over each other, this means
that the hydrogen bonds must be continually breaking and
reforming as each water molecule moves. However
as the temperature drops close to the freezing point of water the movement of the
water molecules slows and
at 40C the density of the water reaches it maximum, then below 40C the water molecules
start to
move apart as more and more hydrogen bonds start to form. The problem is that these hydrogen bonds prefer specific angles. To maximize these angles in the ice lattice structure, the water molecules end up spaced farther apart than they would be in liquid water. This creates the open, airy structure of ice and the water reaches its
freezing point the water molecules will be unable to move because they are held in place
by the hydrogen bonding between adjacent molecules.
In order to form the ice structure the water molecules
are not as densely packed as they are in water, this explains why ice is less dense than water and floats on it.
It is easy to see when you look at the ice structure how open it is. This is a very unusual property since for
most substances the
solid state is denser than the liquid state.
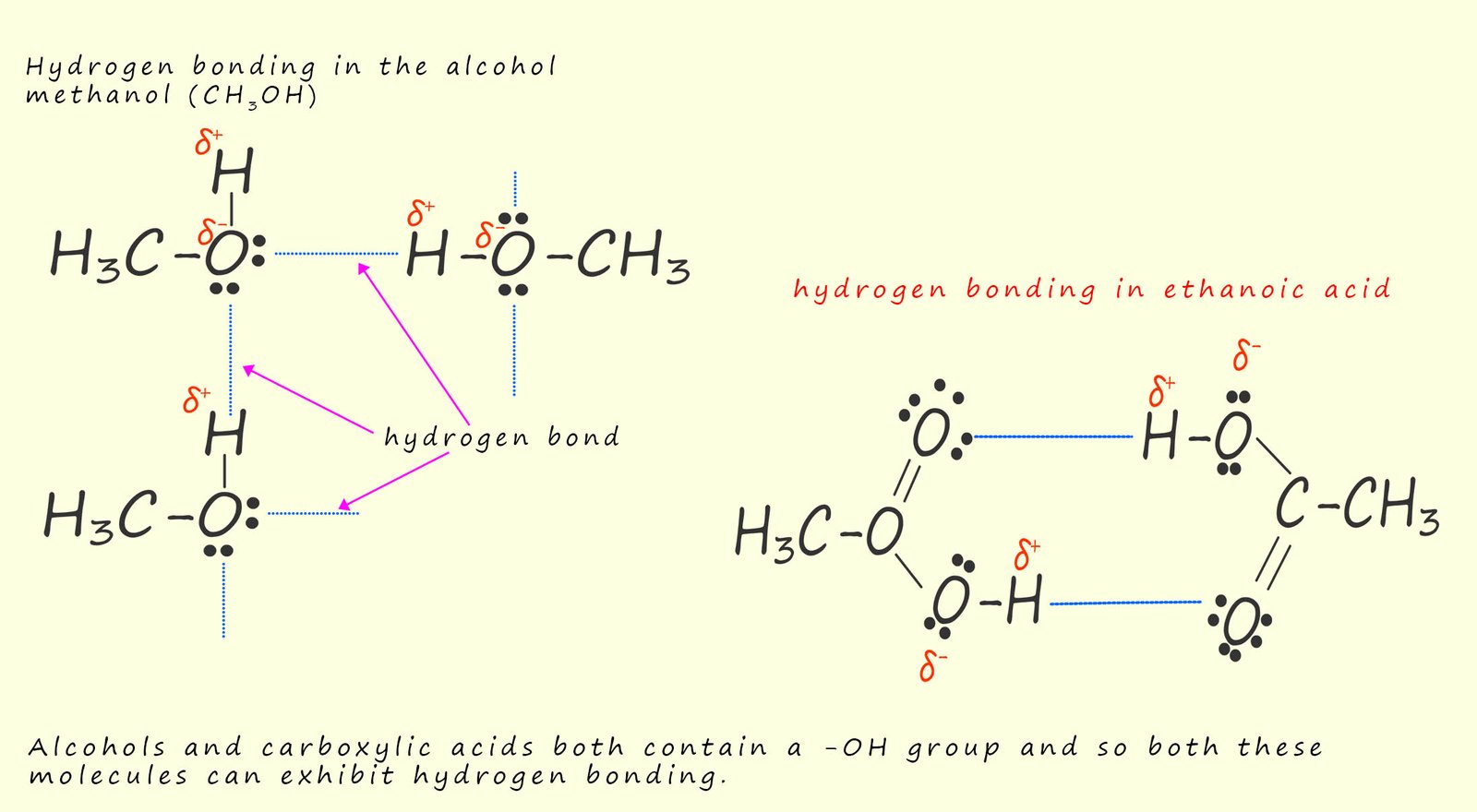
Carboxylic acids and alcohols both contain extensive hydrogen bonding. Most carboxylic acids exist as dimers where two carboxylic acid molecules are attracted to each other and held by 2 hydrogen bonds, this is shown below. This hydrogen bonding in carboxylic acids causes them to have much higher boiling and melting points than expected. Both alcohols and carboxylic molecules can form hydrogen bonds with water; this explains why they are soluble in water but molecules such as alkanes are not. Many organic molecules such as the alkanes are non-polar which means they cannot form hydrogen bonds with polar solvents such as water which is why they are insoluble in water. However for alcohols and carboxylic acids as the molecules get larger and the chain length of the organic part of the molecule increases their solubility decreases.
Hydrogen bonding is critical in maintaining the shape of many important
biological molecules that are essential for life.
These molecules include proteins and DNA.
Proteins consist of long chains of amino-acids
which are arranged in long
helical chains or sheets. These large protein polymers are held in place
by hydrogen bonds between the N-H and C=0 bonds on
different
amino acid molecules within the protein chains or sheets.
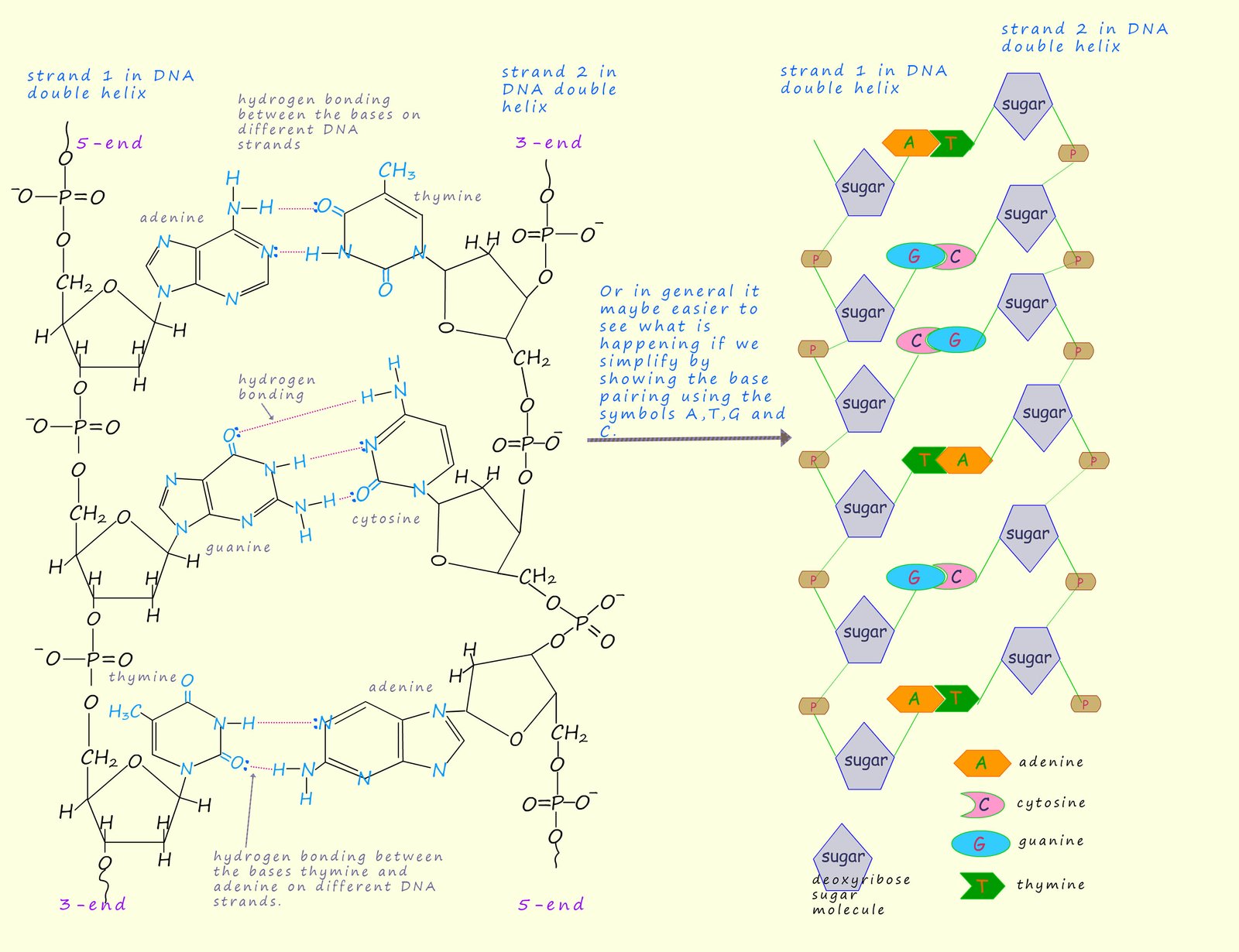
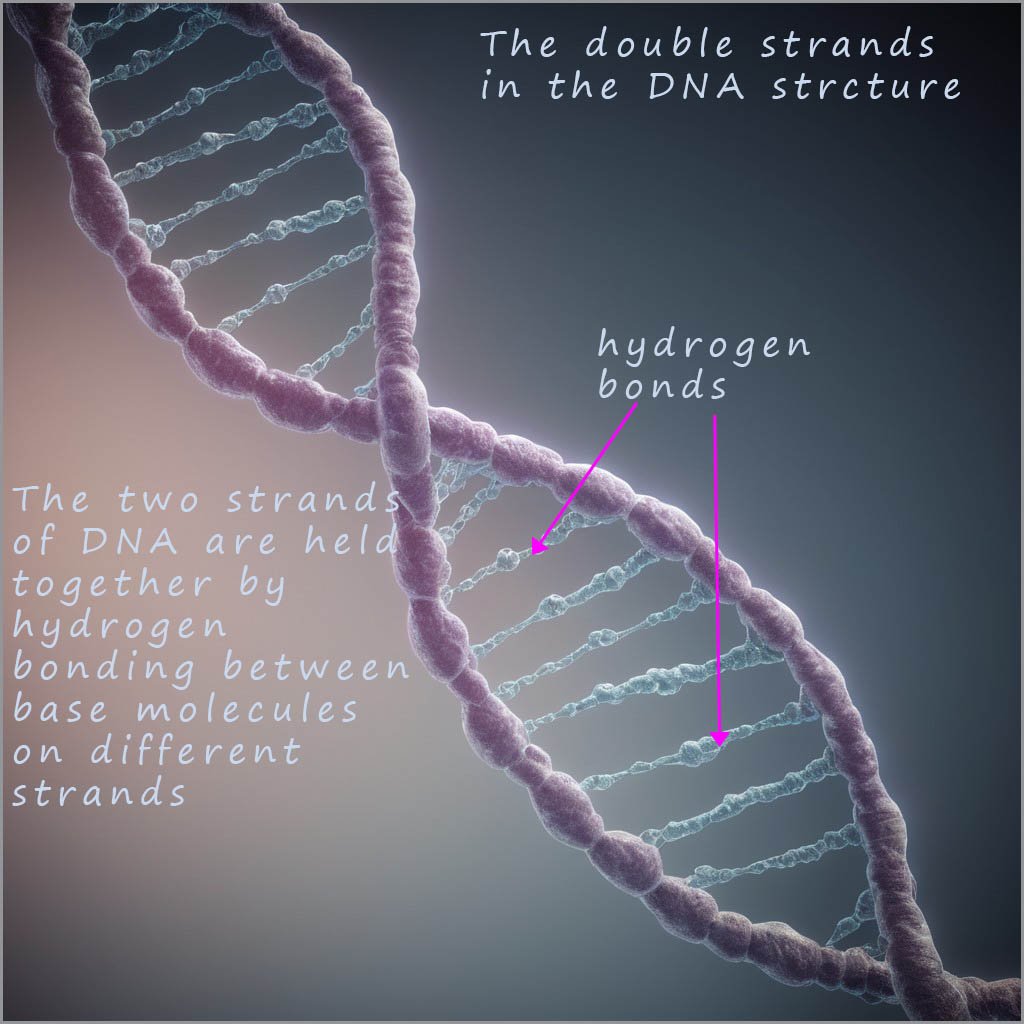
The DNA polymer consists of helical shaped strands which are held together by a series of
hydrogen bonds between 4 bases
in the strands. These bases are called guanine (G), cytosine (C), adenine (A) and thymine (T). Like
the protein polymers these hydrogen bonds occur between N-H groups and C=O groups on the bases in different strands.
In 1953 James Watson and Francis Crick suggested the structure of DNA consisted of two of
polymer strands shown below twisted together in a double helix structure.
The two strands are attracted to each other
through weak intermolecular hydrogen bonding between the bases on each strand. However Watson and Crick
found that the intermolecular hydrogen bonding between the two strands of DNA ONLY occurs between the bases adenine (A) and thymine (T) on different
strands and between guanine (G) and cytosine (C) bases on different strands.
This is shown below where the four bases use hydrogen bonding to link the two DNA strands.