Two amino acid molecules can react with each other in an internal acid-base reaction to form a dipeptide. The diagram below shows how the amino acids alanine and glycine can react with each other in a condensation reaction to form a dipeptide molecule and water.
There are two possible ways these two amino acid molecules can react with each other to form a dipeptide:

The -CONH- group that links the two amino acids in the dipeptide molecule is called a peptide link and the C-N bond within this link is called a peptide bond. Of course the peptide link can also be considered as a N-substituted amide bond. The dipeptide molecule which forms as a result of this condensation reaction still has reactive carboxyl and amino groups on either ends of the molecule and can therefore undergo further condensation reactions with other amino acids to form polypeptides or polyamide molecules. A polypeptide can contain many different amino acids monomers all linked together. Once the polypeptide polymer chains increase to around 50 or more amino acids they are considered large enough to be called proteins. Proteins are large condensation polymers which can have Mr values into the millions.
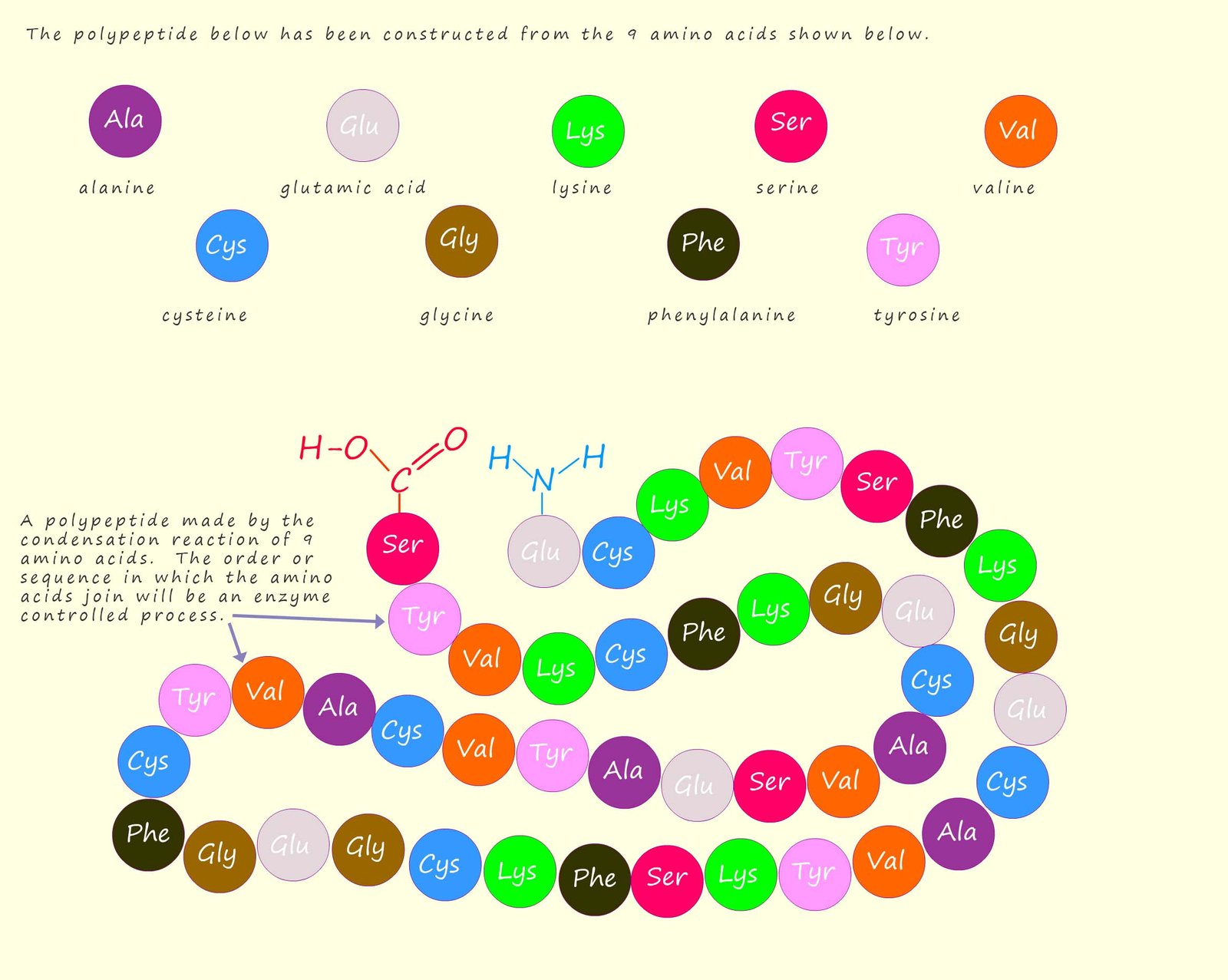
The names of amino acids are often shortened down to a three letter abbreviation or sometimes even a single letter, for example the amino acid glycine can be shortened to "gly" and alanine to "ala". The amino acids that make up polypeptides and proteins are often referred to as amino acid residues. The order or sequence of these amino acids in a polypeptide or protein polymer is referred to as its primary structure, this is outlined in the image below:
However as well as peptide bonds holding together the amino acid residues in a polypeptide chain there is another option if an amino acid called cysteine is present in the polypeptide chain. The amino acid cysteine contains a thiol functional group (R-S-H). This group can form what are called disulphide bridges between cysteine amino acids residues on the same polypeptide chain, this will probably result in the formation of loops in the long peptide chain or it is even possible that these disulfide bridges can operate across different polypeptide chains and this would then link these otherwise separate polypeptide chains together, this is outlined in the image below:

The primary structure of a polypeptide or protein, that is the sequence of the amino acid residues is not the only factor that will influence the ultimate shape of the polypeptide or protein. Proteins are massive molecules with intricate shapes, now these shapes depend largely on the way that the long polymer chains of amino acids fold and orientate themselves in 3d space. The effect of any disulphide bridges between cysteine amino acids can as we have seen led to folds and loops developing in the structure of the protein, but we must also consider the influence of intermolecular hydrogen bonding between the amino group (-NH2) and the carbonyl group (-CO) present in the carboxyl group on the acid end of the amino acid.
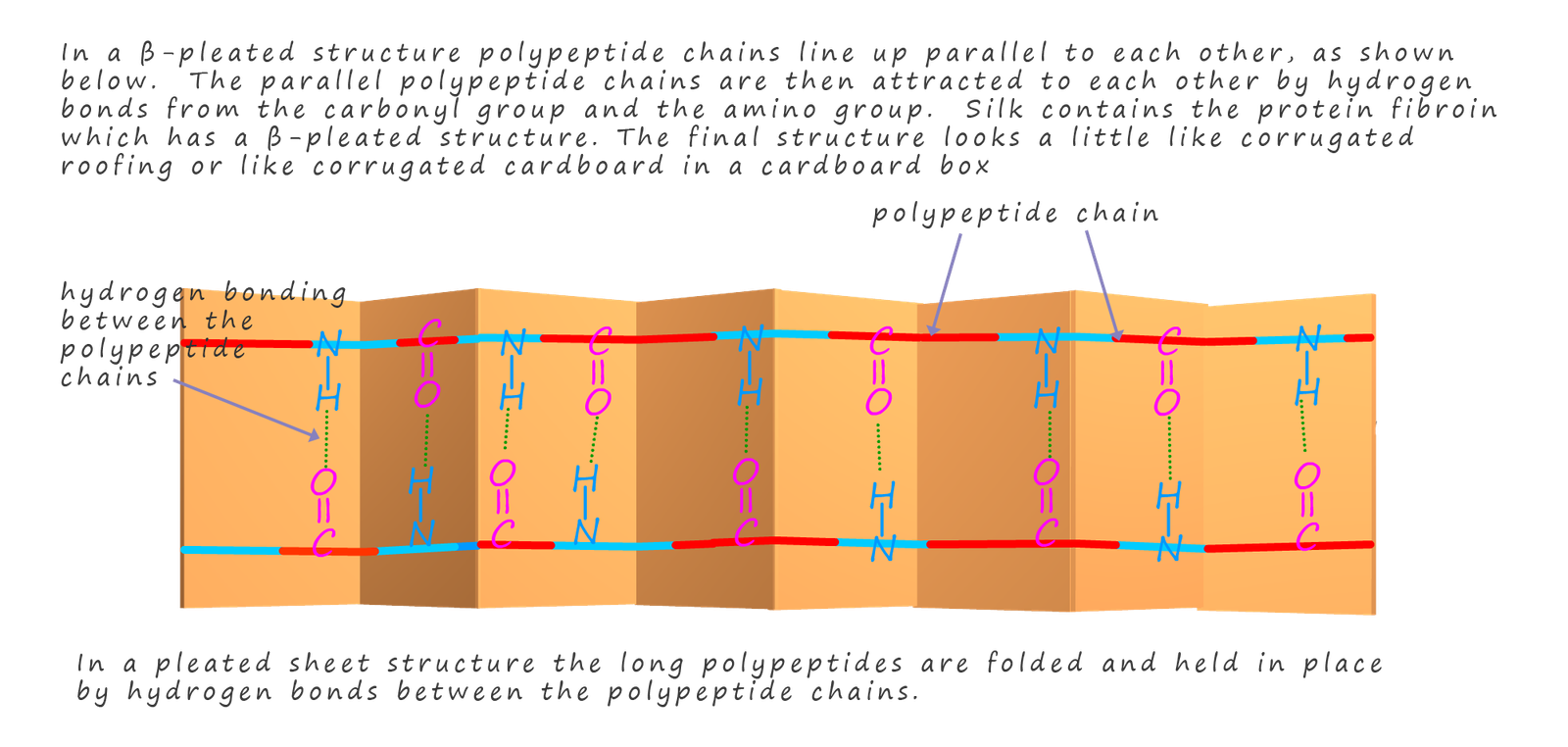
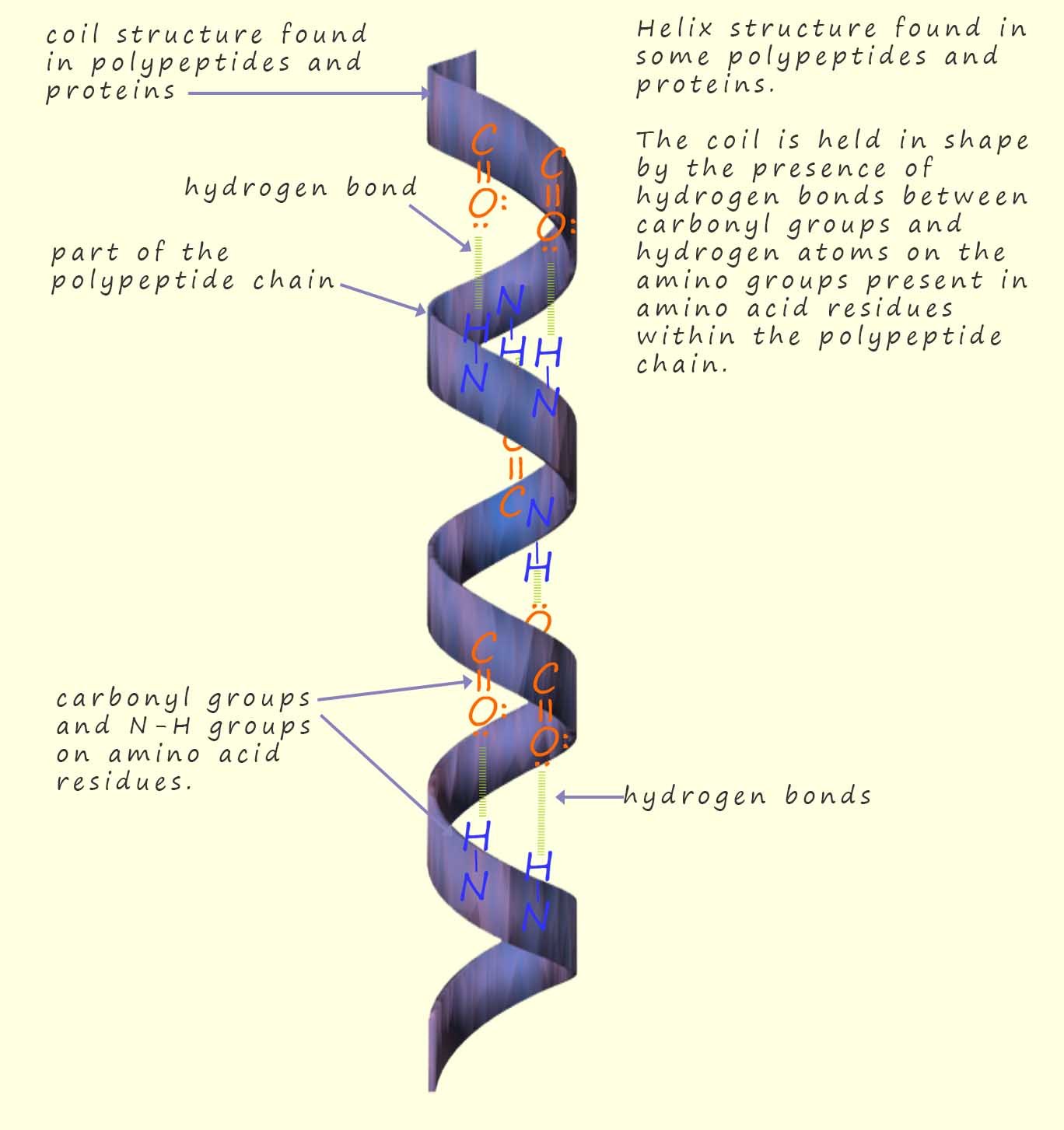
Despite being relatively weak when compared to the strength of covalent bonds, the fact that there is lots of hydrogen bonding present between the amino acid residues in proteins and polypeptide chains will influence the way these long polypeptides fold into their final shape. Two common ways in which long polypeptides chains can fold themselves is as a coil and a structure called a β-pleated sheet, both these structures are shown in the diagrams opposite and below.
The influence of hydrogen bonding here is self-evident from the diagrams in helping to hold the long peptide chains in place. The shape in which segments or parts of the backbone of long polypeptides fold themselves is called the secondary structure of the protein. While the tertiary structure refers to the way in which the whole polypeptide chain folds and orientates itself in space to form an overall 3d shape for the protein.
The β-pleated sheet structure for polypeptides chains is not as common as the coiled structure, it occurs when amino acid chains fold to give parallel chains of polypeptides which bond to each other using hydrogen bonding across the peptide links in different polypeptide chains. This type of structure is found in silk, where the protein fibroin is composed mainly of this type of β-pleated sheet structure.