
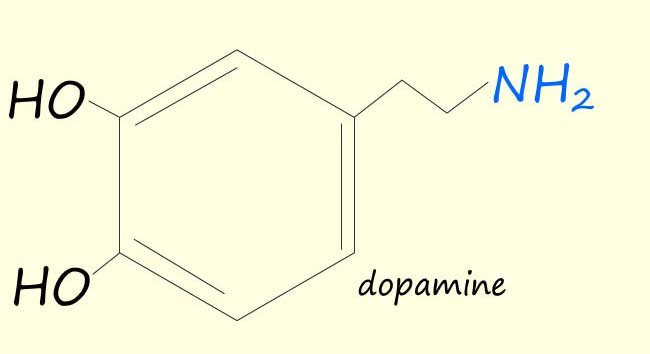
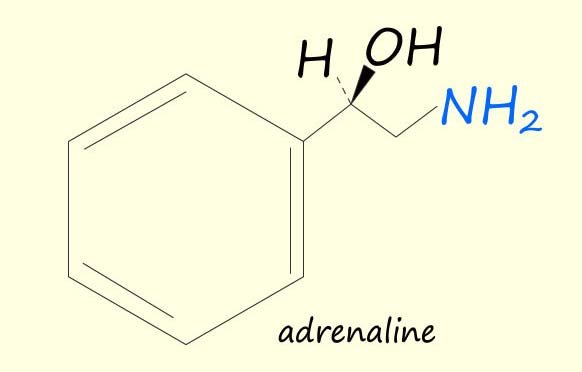
Amines are found in a wide variety of living organism including plants and animals. Many amines have physiological effects on the body e.g. dopamine, adrenaline and amphetamine are three amines that you will probably have heard of each of which has a large physiological effect on the human body.
| dopamine | adrenaline | amphetamine |
|---|---|---|
 |
 |
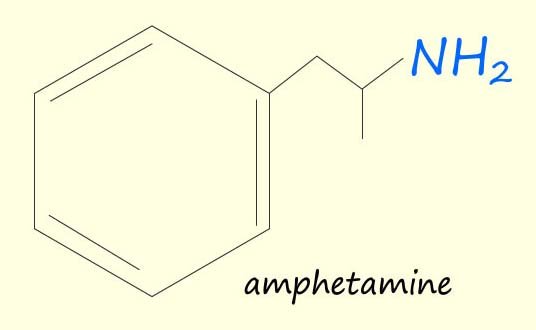 |
| Dopamine is a crucial neurotransmitter involved in various bodily functions. It plays a key role in movement, coordination, and the experience of pleasure, driving motivation and reward-seeking behaviour. Produced in the brain, it's released during enjoyable activities or in response to stress. | Adrenaline is a hormone released in the adrenal glands and is responsible for the body’s “flight or fight” response. Adrenaline prepares your body to react to stress or a dangerous situation. It Increases: heart rate and blood pressure, breathing rate, blood flow to the muscles, and it increases alertness and reduce the sensation of pain. | Amphetamines are stimulant drugs. They increase the activity in the brain and central nervous system. They give you a feeling of euphoria. They also increase your alertness, heart rate and blood pressure. |
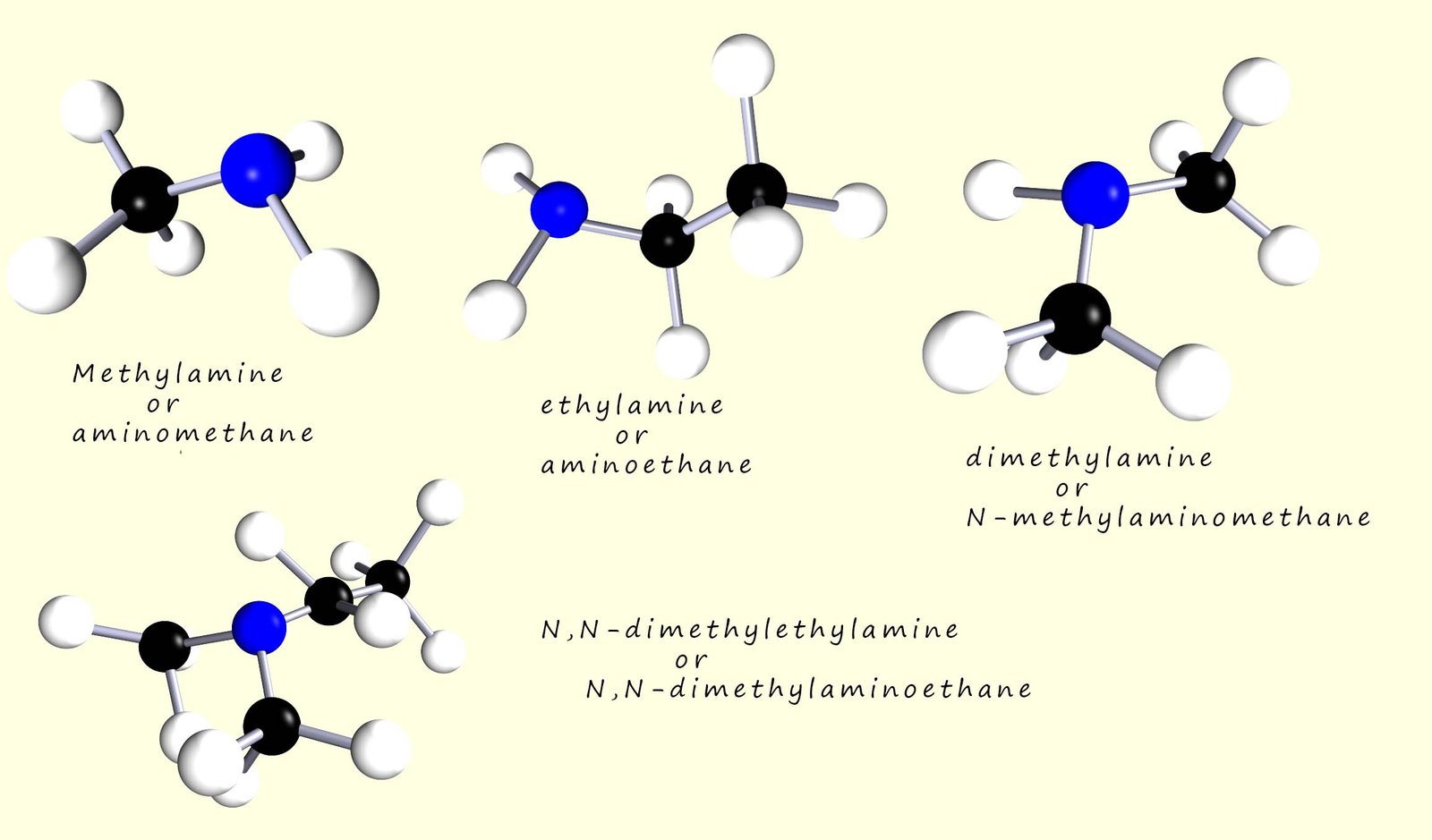
Amines can be thought as substituted ammonia molecules, where one or more of the hydrogen atoms on the ammonia molecule have been replaced by an alkyl group. Amines are classified as either primary, secondary or tertiary depending on the number of hydrogen atoms in an ammonia molecule that have been replaced by an alkyl group for example the image below shows example the image below shows a primary, secondary and tertiary amine.
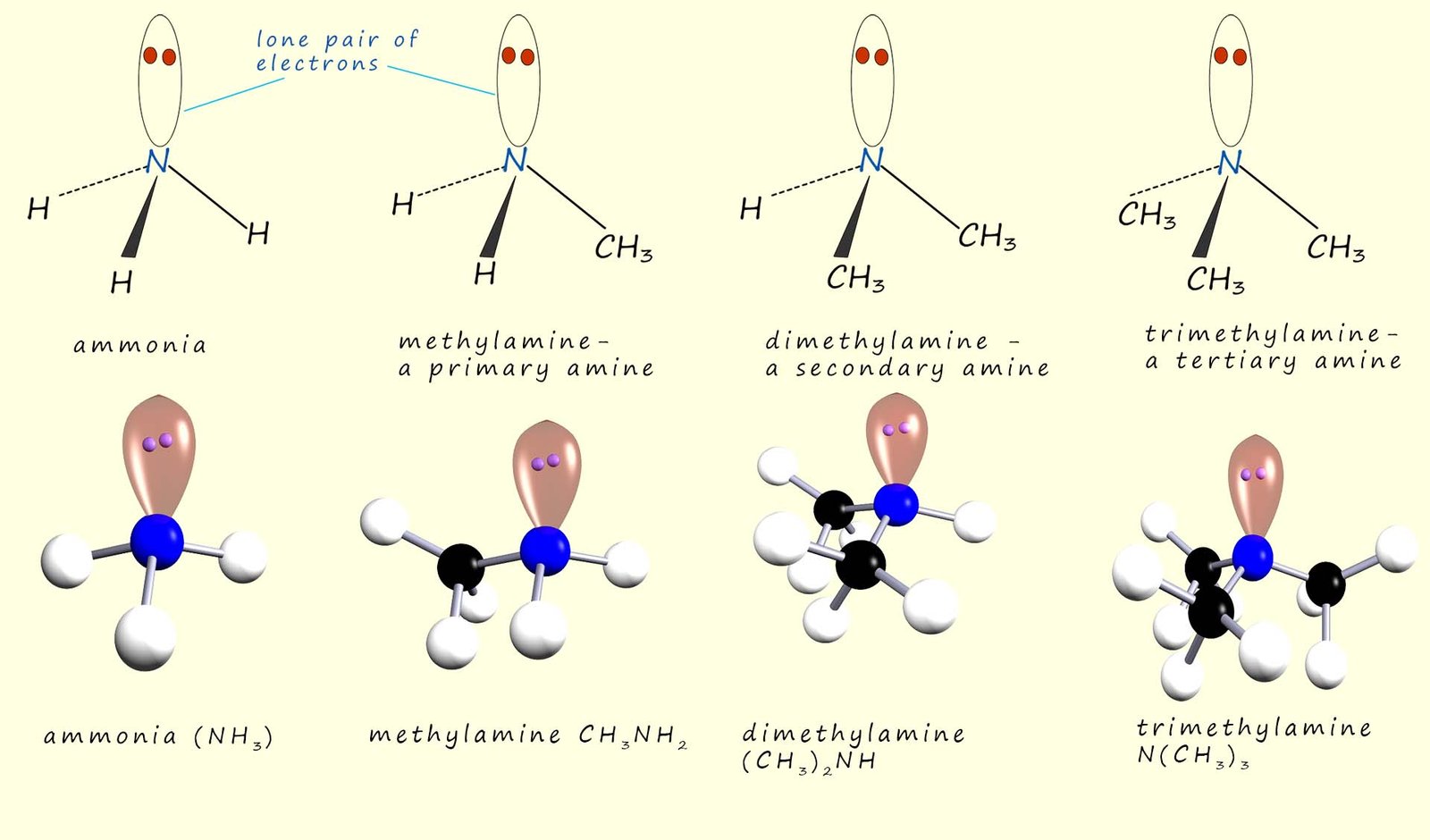
In a primary amine one of the hydrogen atoms in an ammonia molecule has been replaced by an alkyl group; a methyl group (-CH3) in the image above. In a secondary amine two of the hydrogen atoms in an ammonia molecule have been replaced by alkyl groups whereas in a tertiary amine all three hydrogen atoms in an ammonia molecule have been replaced by an alkyl group.
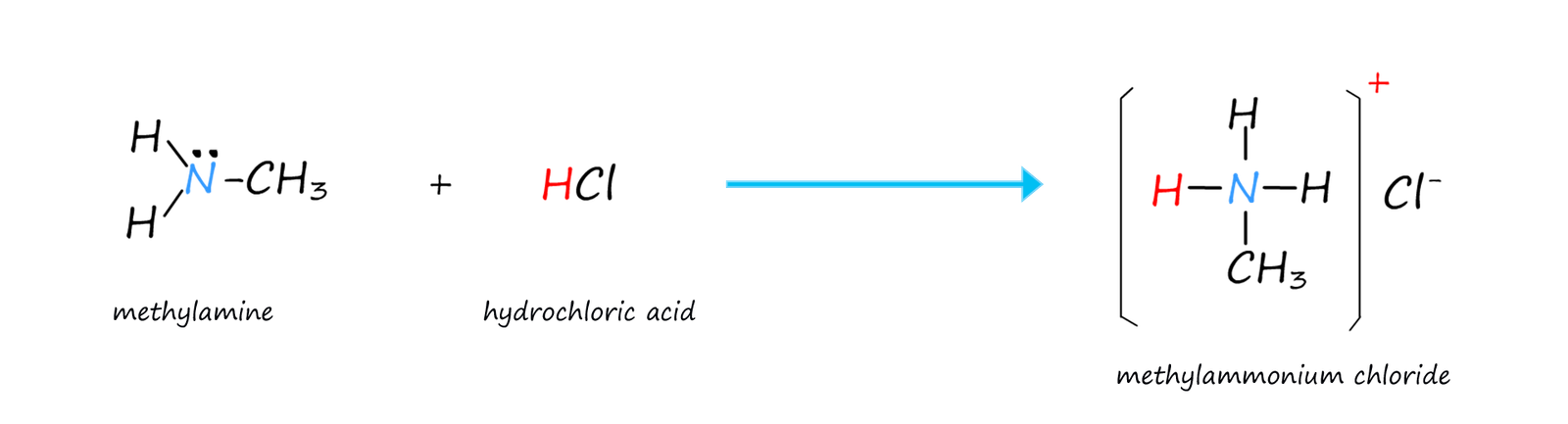
In all the amines shown above the nitrogen atom makes 3 covalent bonds and has one lone pair of electrons. However it is also possible for the nitrogen atom to form four covalent bonds. In this case an ionic compound is formed. The nitrogen atom can use its lone pair of electrons to form a dative covalent bond, for example ammonia and amines can act as Lewis bases and accept a hydrogen ion (H+) from an acid, this is outlined in the image below:
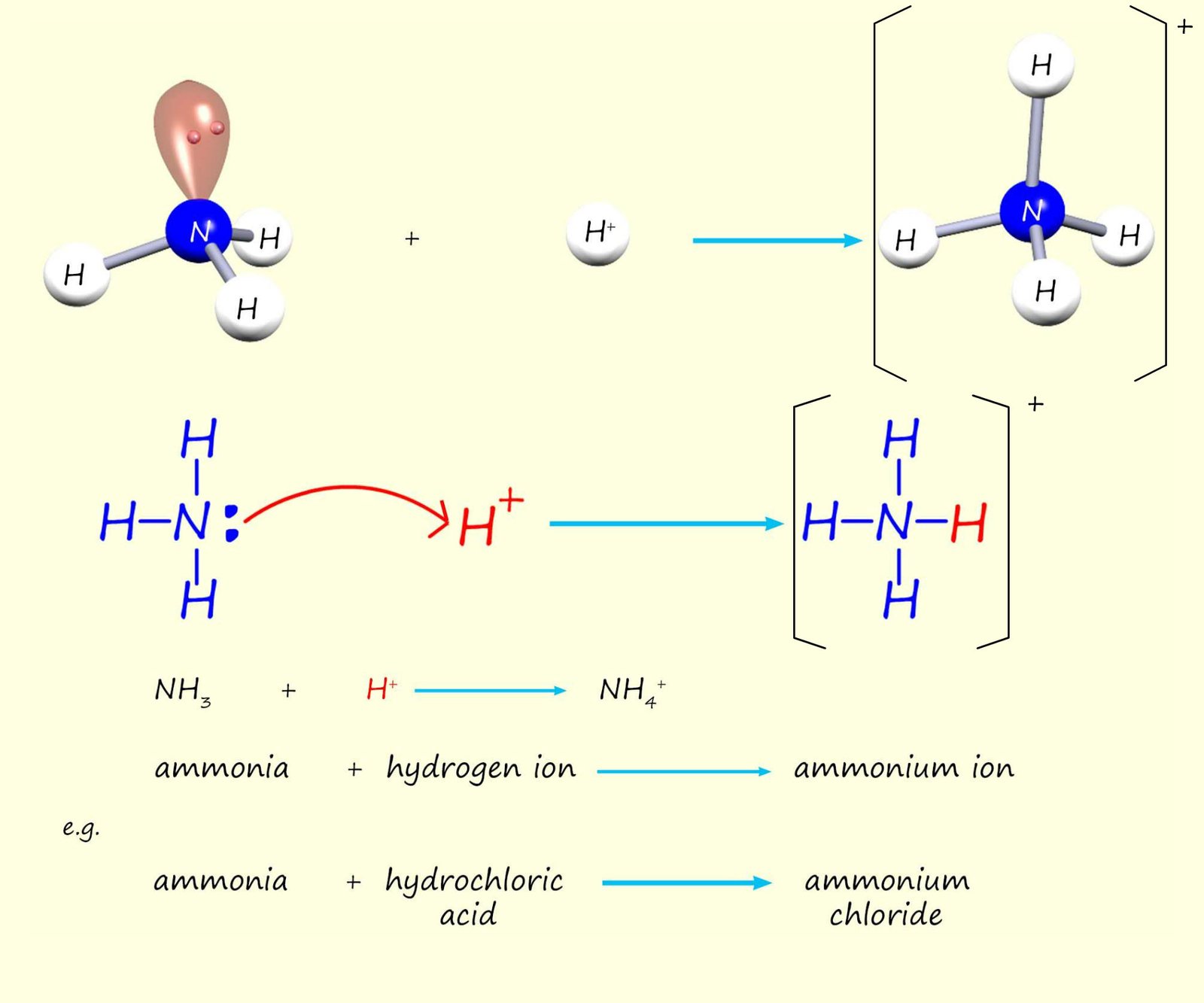
The ionic compounds formed fall into two distinct groups:

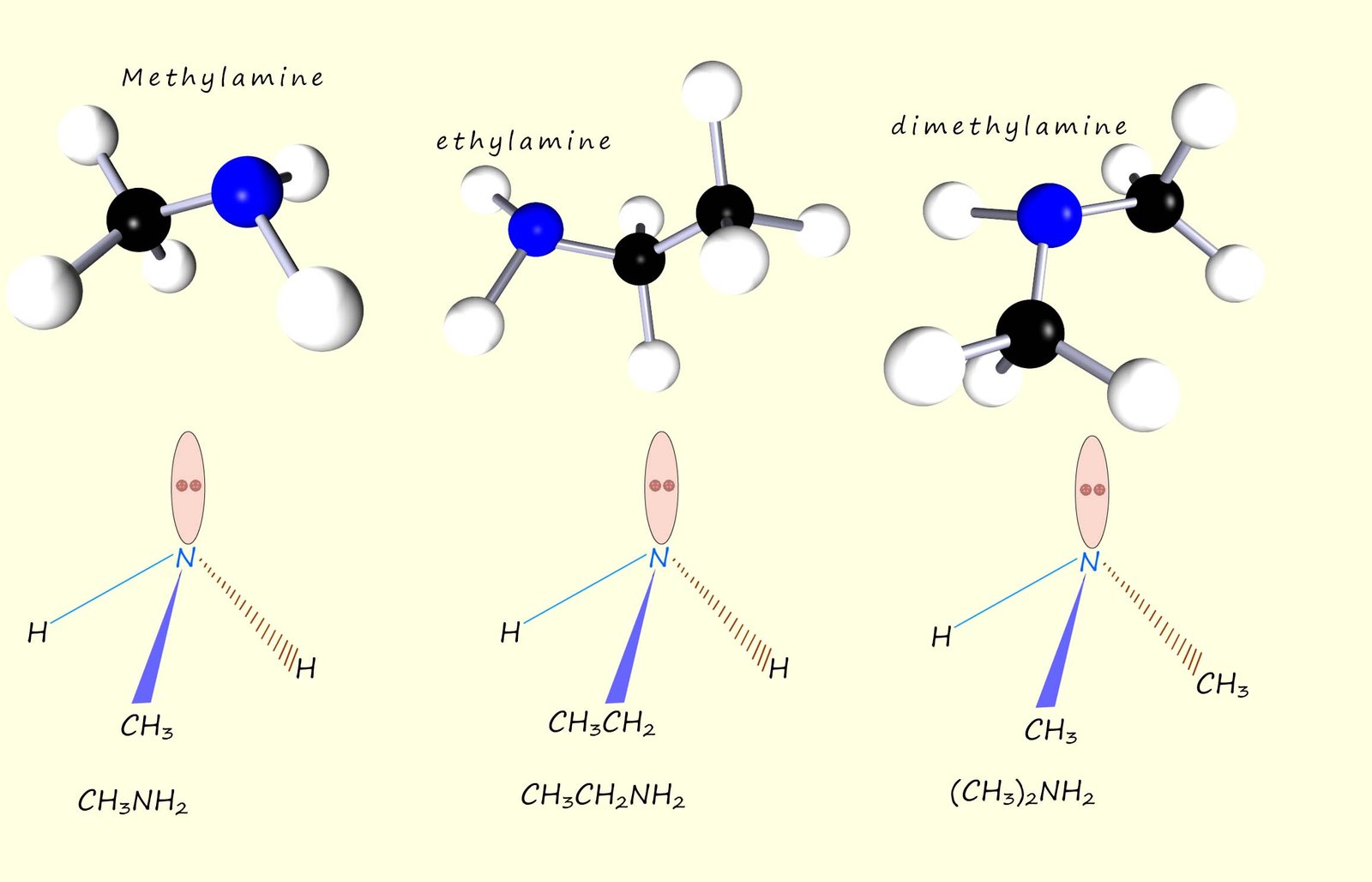
Naming amines can be a bit of a nightmare at times as there are several naming systems in use but luckily most exam boards seem to prefer the traditional naming system I have outlined below. Simple amine molecules are named by simply named by adding the suffix -amine to the name of the alkyl or aryl group; for example the molecules below can be thought of as simply an ammonia molecule where one or more of the hydrogen atoms has been replaced by an alkyl group.
If more than one alkyl group is attached to the nitrogen atom then the largest alkyl group is chosen as the parent name and the other alkyl group is assigned as a N-Alkyl group, for example in each of the molecules shown below the largest alkyl chain is a propyl chain (C3H7), so this will be the parent name of the amine. The other substituent(s) are labelled as N-alkyl substituents to indicate that they are attached to the nitrogen atom:
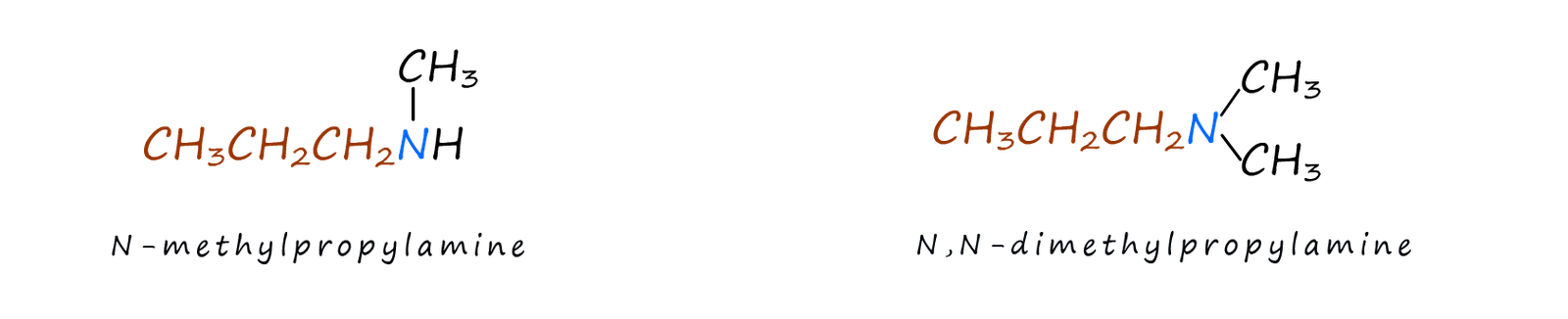
You may come across another method used to name amines whereby instead of using the suffix -amine to name the compounds instead the prefix -amino is used; the image below gives several examples of this naming system. However most exam boards seem to prefer the traditional naming system above so I would use this unless your teacher tells you otherwise.
So for example simply use the prefix amino in front of the name of the longest alkyl chain present in the molecules below. The first two examples are primary amines while the third example is a secondary amine. Here use the same rule, identify the longest alkyl chain which will be the parent name and other substituents will be labelled as N-substituents, although you will also many examples where the "N" is simply dropped altogether when you use the -amino naming system.
The images below show a number of amine salts and a quaternary ammonium salt. The naming of these salts is straightforward. The 10, 20 and 30 amines form 10, 20 and 30 ammonium salts while tertiary amines (30) form quaternary ammonium salts:


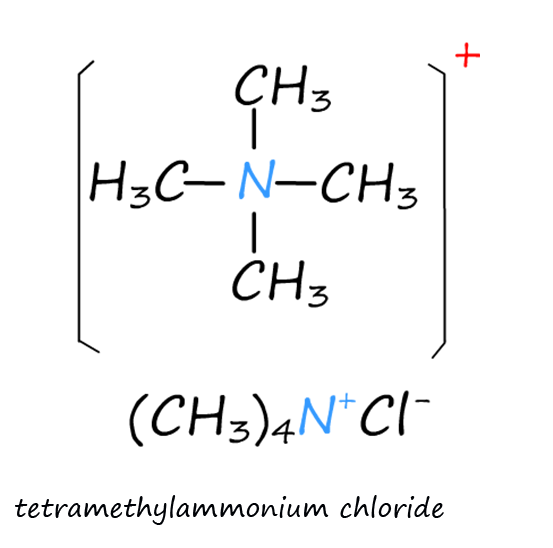
Quaternary ammonium salts form when an alkyl substituent adds to a tertiary amine, tetramethylammonium chloride (shown opposite) would be an example of a quaternary ammonium salt.
Quaternary ammonium salts have many uses including:

Amines molecules contain a N-H bond and so will undergo hydrogen bonding. The hydrogen bonding in amines is weaker than in water or alcohols since the N-H bond is not as polar as the O-H bond. This means that for example the boiling points of amines are higher than similar non- hydrogen bonded molecules such as alkanes but lower than similar molecular weight alcohols.
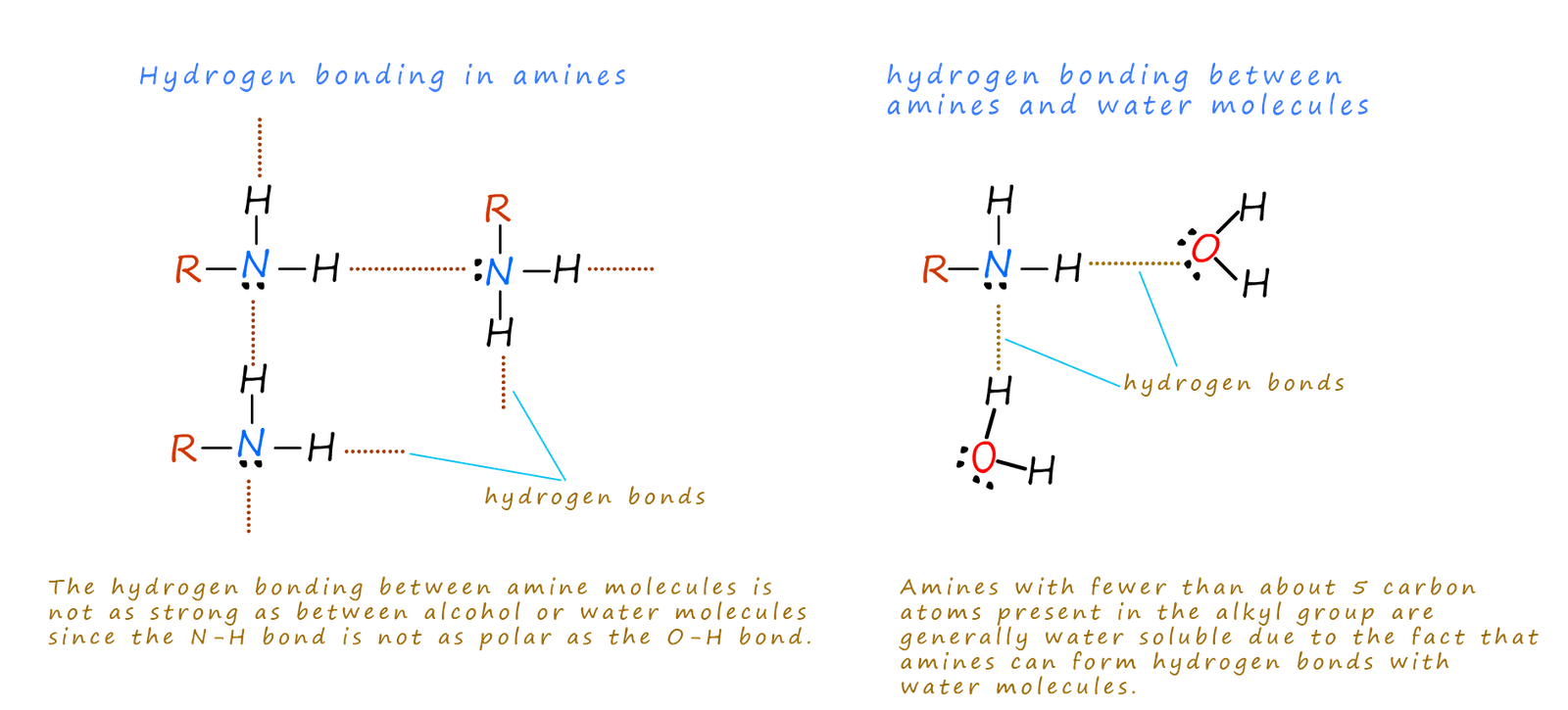
Since tertiary amines do not have any hydrogen atoms attached directly to the nitrogen atom they cannot undergo any hydrogen bonding and as a result tertiary amines have lower boiling points than comparable primary and secondary amines.
Amines are perhaps best known for their rather foul smelling odours, most have a fishy odour but many especially diamines have a rather putrid smell of decaying matter. The chemistry of ammonia and amines is largely dominated by one aspect of their structure- the lone pair of electrons on the nitrogen atom. The presence of this lone pair of electrons makes amines excellent Brønsted-Lowry bases (H+ or proton acceptors). Ammonia and amines can form dative covalent bonds with hydrogen ions (H+) to form ammonium salts, as shown above:
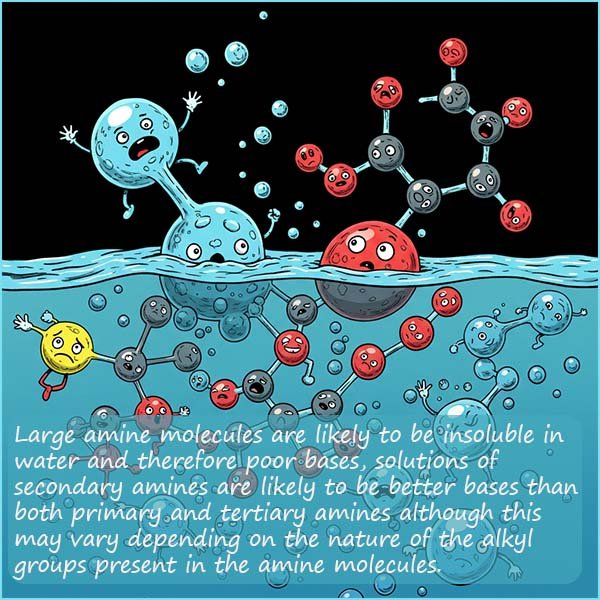 The presence of an alkyl substituent on an ammonia molecule increases the base strength due to the positive inductive effect of the alkyl group which will push electron density onto the nitrogen atom. Therefore in general secondary amines will be stronger bases than primary amines and tertiary amines will be stronger bases than secondary amines. Although this trend is really only observed when the amines are in the gaseous state.
The presence of an alkyl substituent on an ammonia molecule increases the base strength due to the positive inductive effect of the alkyl group which will push electron density onto the nitrogen atom. Therefore in general secondary amines will be stronger bases than primary amines and tertiary amines will be stronger bases than secondary amines. Although this trend is really only observed when the amines are in the gaseous state.
In aqueous solutions the trend in base strength for amines is usually secondary > primary > tertiary although the exact trend will depend on the alkyl groups present in the amine molecules. The reason for this reversal for tertiary amines is steric hindrance and solvation effects. Tertiary amines, while having the most positive inductive effect is also the most sterically hindered. This makes it harder for a water molecule to solvate the resulting ammonium ion produced. The solubility of the amine will also obviously influence its ability to act as a base and as mentioned above amines with more than about five carbon atoms present in the molecule are likely to be insoluble in water.
However aromatic amines, such as phenylamine or aniline are poor bases. In an aromatic amine the nitrogen atom is bonded directly to an aromatic ring, this means that the lone pair of electrons on the nitrogen atom can be delocalised through the aromatic ring and so are not readily available to form dative covalent bonds in the same way aliphatic amines can. This is outlined in the diagram below:
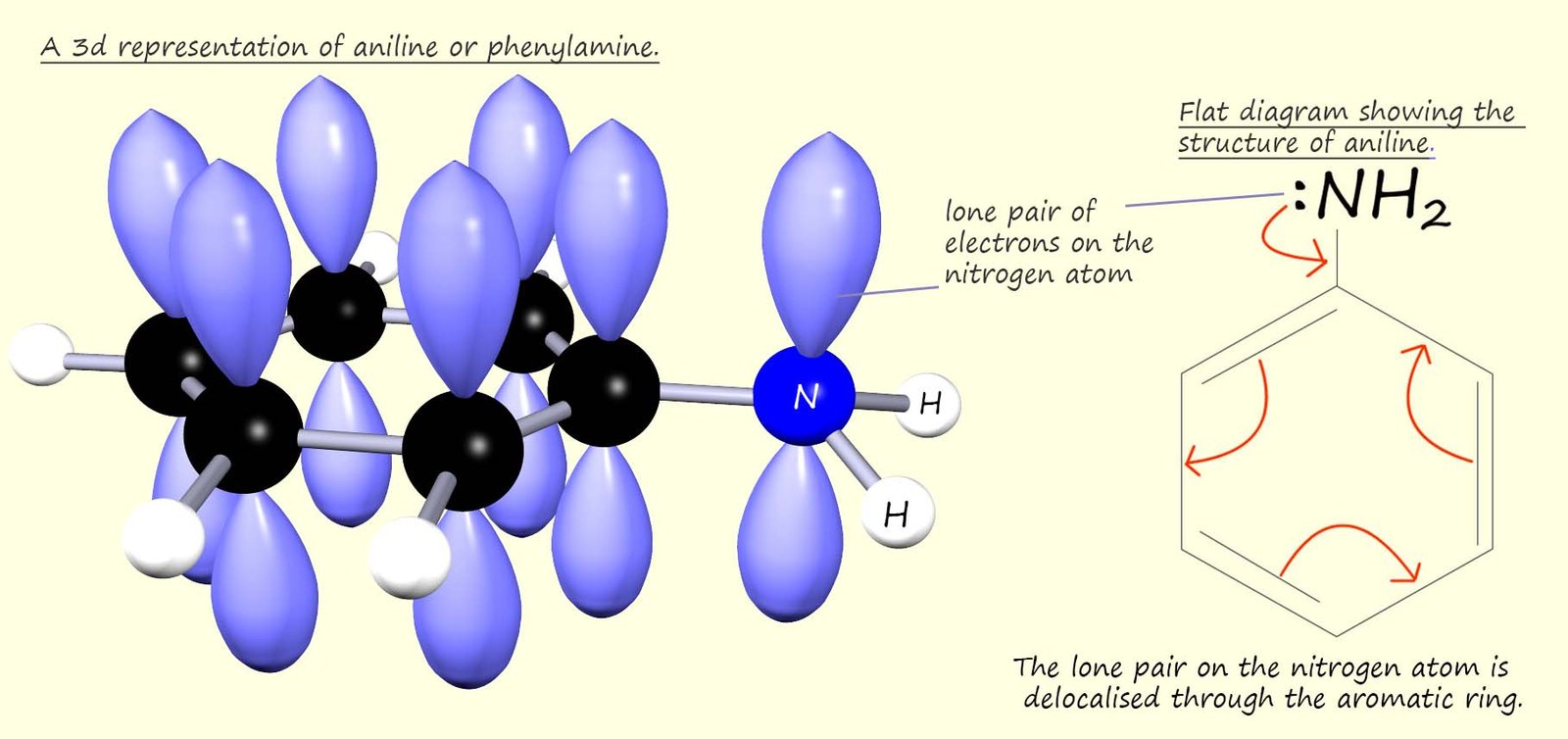
In aniline the NH2 group is attached to an aromatic ring. The aromatic ring contains 6 delocalised electrons, one from each carbon atom in the ring. The p-orbitals in the ring can merge with the lone pair electrons on the nitrogen atom, which are also in a p-orbital. This means that the lone pair on the nitrogen atom is delocalised through the aromatic ring system and not available to form dative covalent bonds, that is aromatic amines are poor bases because the nitrogen lone pair is delocalised through the aromatic ring.