
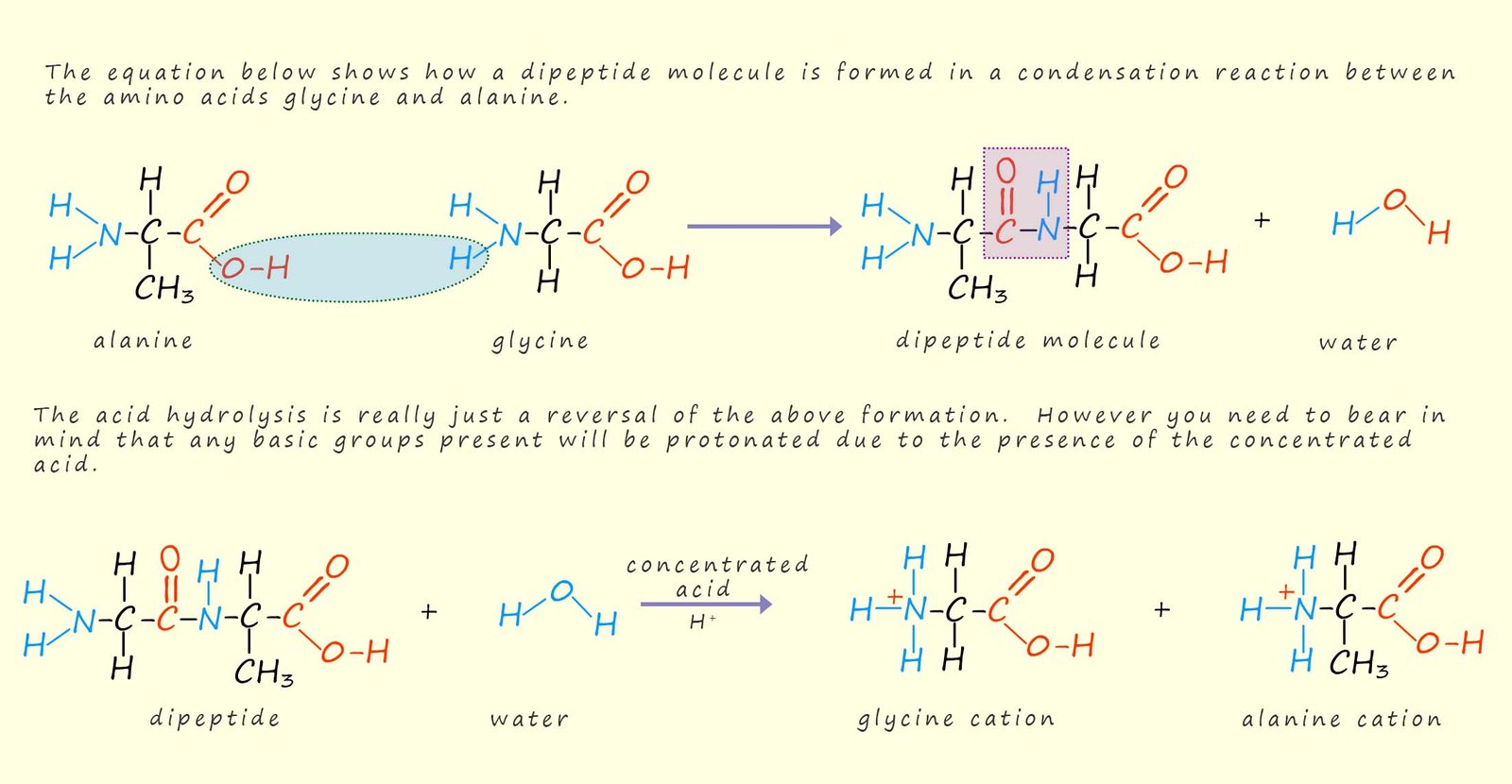
Hydrolysis the breaking down of a substance using the elements of water. In the lab hydrolysis is usually carried out by heating the substance to be broken down with concentrated acid (H+) or concentrated alkali (OH-) under reflux for several hours. Under acidic conditions a polypeptide or protein will be broken up into numerous amino acids that make up the polypeptide or protein. Obviously if the hydrolysis reaction is carried out in the presence of concentrated acid the carboxylate ion (-C02-) will be protonated as will the amine group present in the amino acid. This results in the formation of a cation. As a simple example the acid hydrolysis of the dipeptide formed from the amino acids alanine and glycine, this is outlined in the image below:
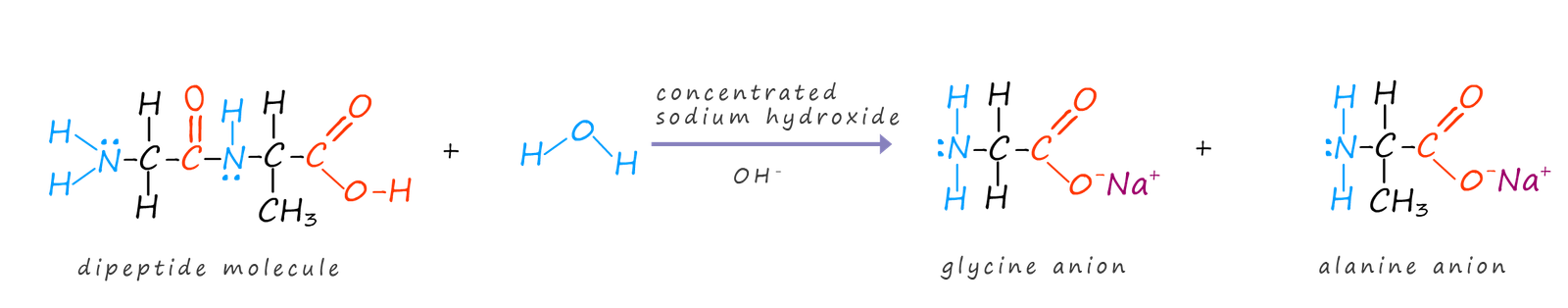
Polypeptides and proteins can also be hydrolysed by heating them under reflux with a concentrated alkali such as sodium hydroxide. This time the amino acids produced will react with the alkaline sodium hydroxide to form the sodium salt from the carboxyl group on the amino acid. This is outlined below where a dipeptide is being hydrolysed.