Amino acids are the monomers used to make proteins. There are many different types of proteins found inside living organisms from the collagens found in tendons and arteries to the keratin in hair, nails and animal hoof. Enzymes, the biological catalysts used in living organisms to control many essential bodily functions also consist of proteins.
Amino acids are the small monomer units that are used to build the larger protein polymer molecules. As the name implies amino acids contain two functional groups; the basic amino group (-NH2) and the acidic carboxyl group (-COOH).
Around 20 amino acids are found inside the human body, they are all α-amino acids (that is they have the basic amino and the acidic carboxyl groups attached to the same carbon atom). With the exception of glycine all the amino acids are optically active molecules with two different enantiomeric forms for each amino acid, however in nature only the S-enantiomer for each amino acid is used in building up proteins and polypeptide molecules. It is quite remarkable to think that from these 20 amino acids that the huge range and types of proteins found in living organism can be synthesised, but think how many words can be made from the 26 letters in the alphabet! The structure of four common α-amino acids found in the human body are shown in the diagram below:
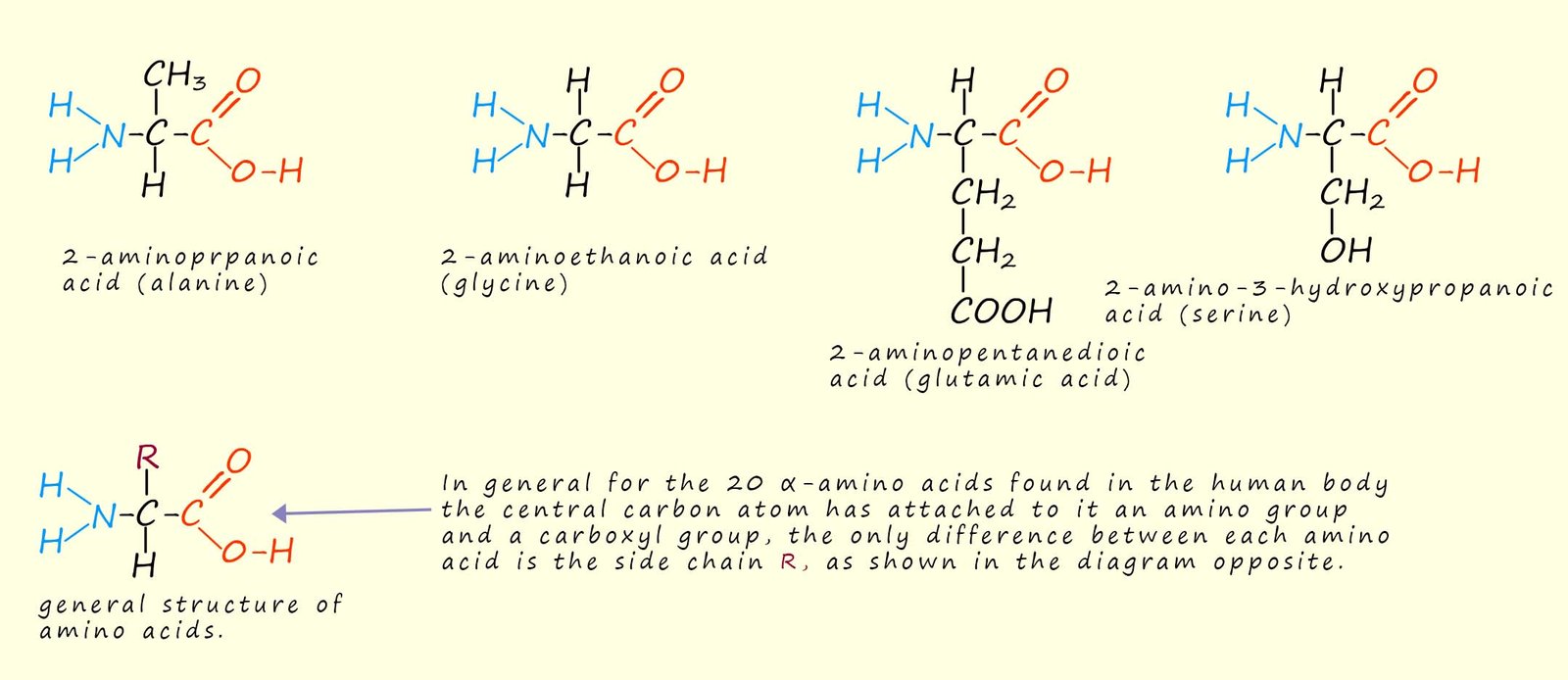
I am sure you may have noticed a problem with the structures of the amino acids drawn above. How can we have a molecule with an acidic group on one end and a basic group on the other end? Surely they will just neutralise each other in an acid-base reaction? Before we answer that question here a few facts that might help with an answer to this question!
Amino acids do not behave as you might expect a typical organic compound to, for example:

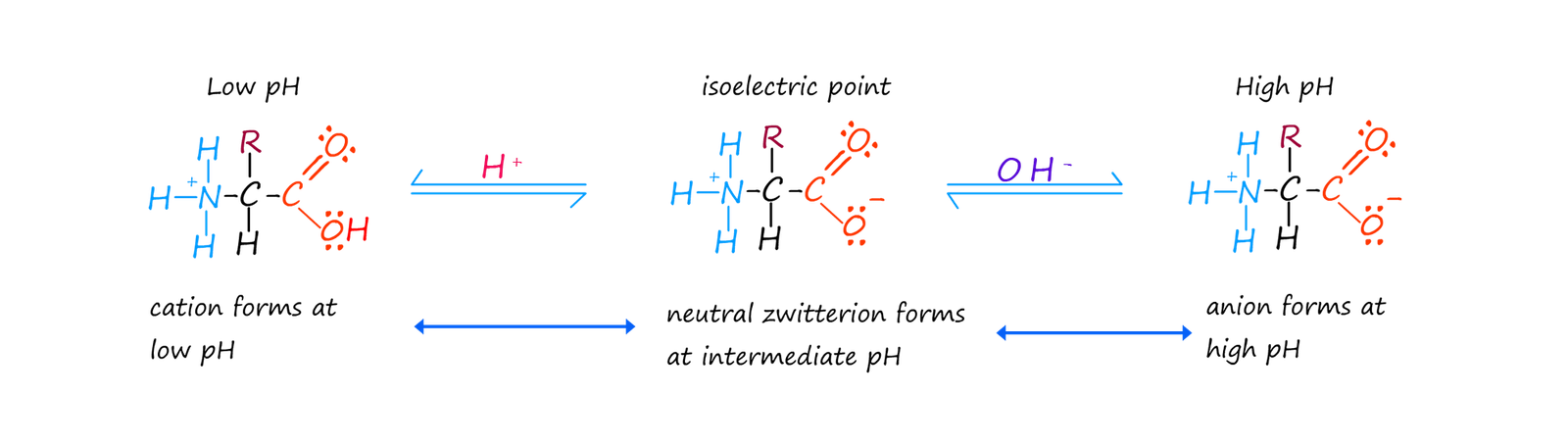
During this interal acid-base neutralisation reaction a hydrogen ion (H+) is transferred from the acidic carboxyl group to the basic amino group in the alanine molecule. The zwitterion has a negatively charged carboxylate anion at one end of the molecule and a positively charged ammonium ion at the other end, but overall the zwitterion has no overall charge since the positive and negative charges present on the ion simply cancel each other out.
The zwitterion ion of an amino acid contains acidic and basic groups. Though perhaps not the groups you were expecting! The basic group is the carboxylate anion (-COO-) which is the conjugate base of the carboxylic acid (-COOH) and the acidic group is the ammonium ion (-NH3+), which is the conjugate acid of the amine group (-NH2). Since it contains both acidic and basic groups the amino acid zwitterion ion is amphoteric, that is it can react with both acids and bases.

To summarise: If the zwitterion is:
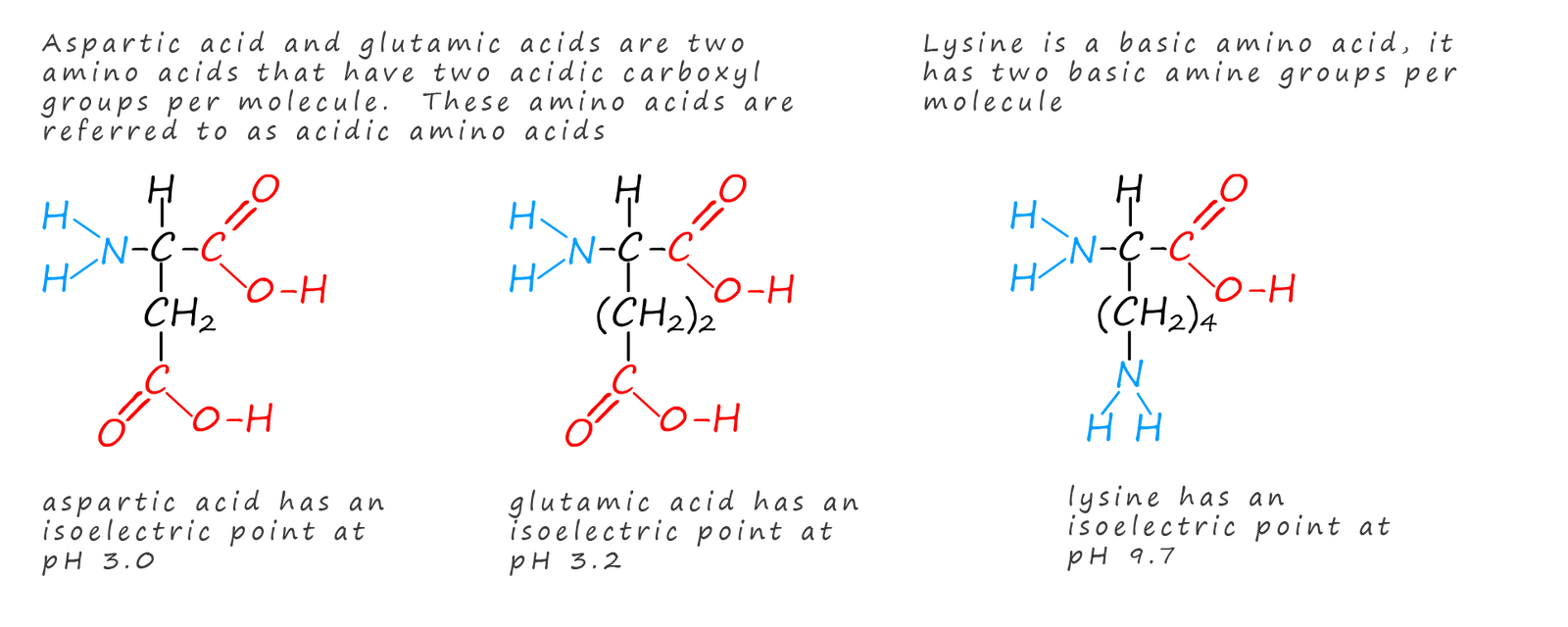
A common misconception among students is that the isoelectric point will be at pH 7, but this is not the case. The acidic ammonium group (-NH3+) is a stronger acid than the basic carboxylate group. You must also consider the nature of the side chain R on the amino acid. In some amino acids this side chain may contain acidic and basic groups which will affect the pH at which the neutral zwitterion exists as a neutral ion. However for most amino acids the isoelectric point is around pH 5-6.5.