
Optically active molecules
rotate plane polarised light. One
enantiomer will rotate
plane polarised light
anti-clockwise while the other enantiomer
will rotate
plane polarised light clockwise.
The enantiomer that rotates
plane polarised light anti-clockwise is said to be
laevorotatory while the enantiomer that rotates plane polarised light
clockwise is said to be dextrorotatory.
By convention anti-clockwise rotation is also give the minus
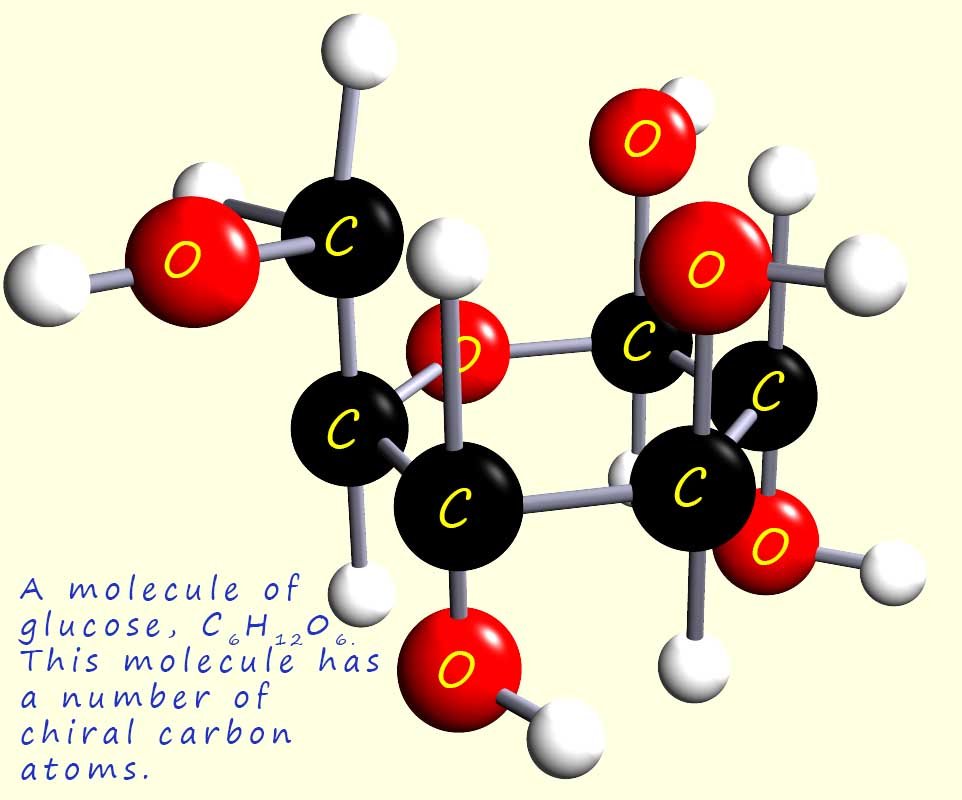
sign (-) while clockwise rotation is given the positive sign (+) e.g. glucose (shown opposite)is an
optically
active molecule, (+)-glucose is dextrorotatory
and will rotate plane polarised light clockwise,
while (-)- glucose is laevorotatory and will
rotate plane polarised light
anti-clockwise.
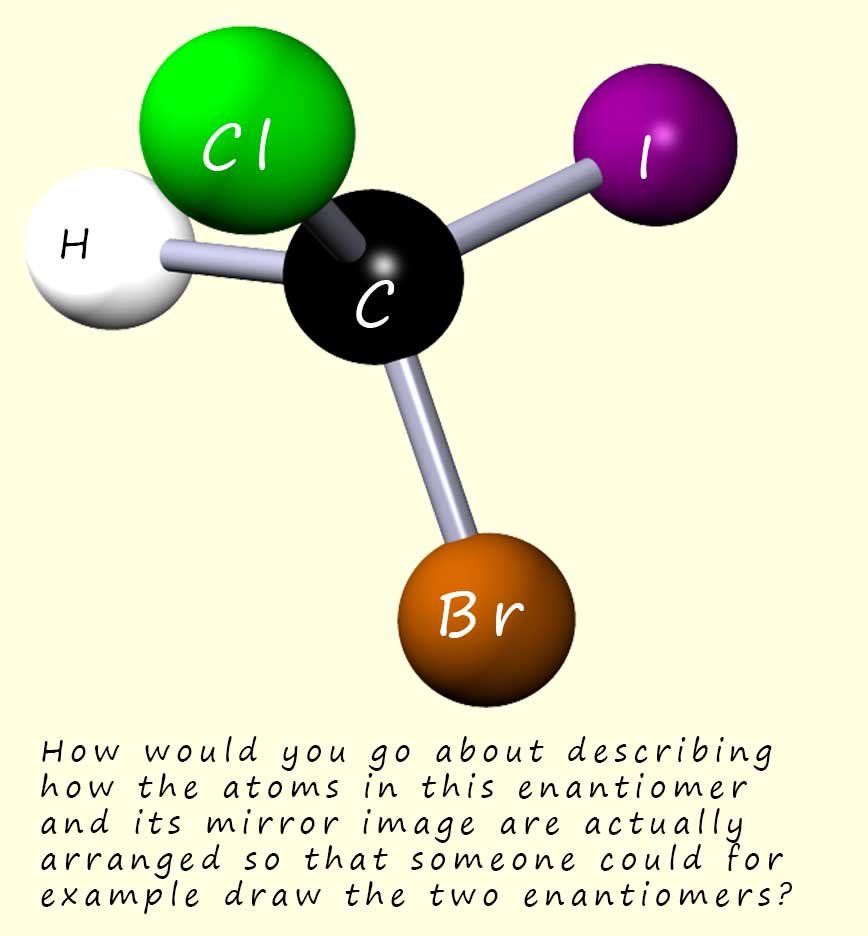
The molecule shown opposite contains a
chiral carbon atom bonded to four different atoms (it could also be groups), so this
molecule will be
optically active and consist of a pair of enantiomers.
We can place a sample of each enantiomer in a
polariser and find out which of the
enantiomers rotates
plane polarised light clockwise and which
enantiomer rotates
plane polarised light anti-clockwise. However this will
not give any
indication of how
the attached atoms are actually arranged around the chiral
carbon atom.
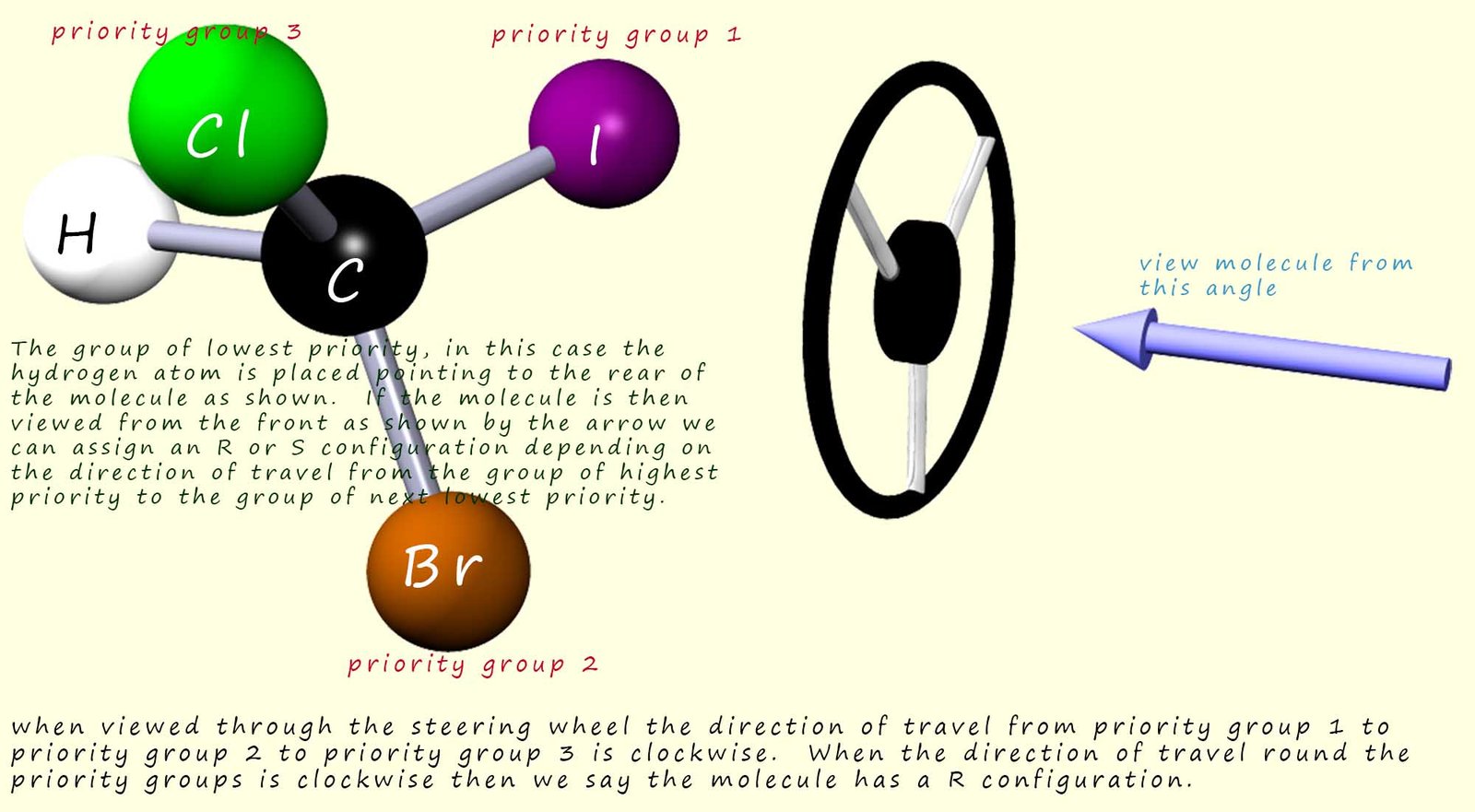
This is where we use the
Cahn-Ingold-Prelog
rules to help use assign orders of priority for each of the attached groups.
Once the orders of
priorities of the groups attached to the chiral carbon have been assigned then we can
determine the relative configuration of the attached groups. These
relative configurations are labelled simply
as R and S. It is very easy and straightforward to assign a R and S configuration to an
optically active molecule.
Simply follow these simple rules:
The image below shows the mirror image or the other enantiomer of the molecule above. You can see that this time when we travel from high priority atoms to lower priority atoms attached to the central chiral carbon atom we travel in an anti-clockwise direction. This tells us that this molecule has a S-configuration.
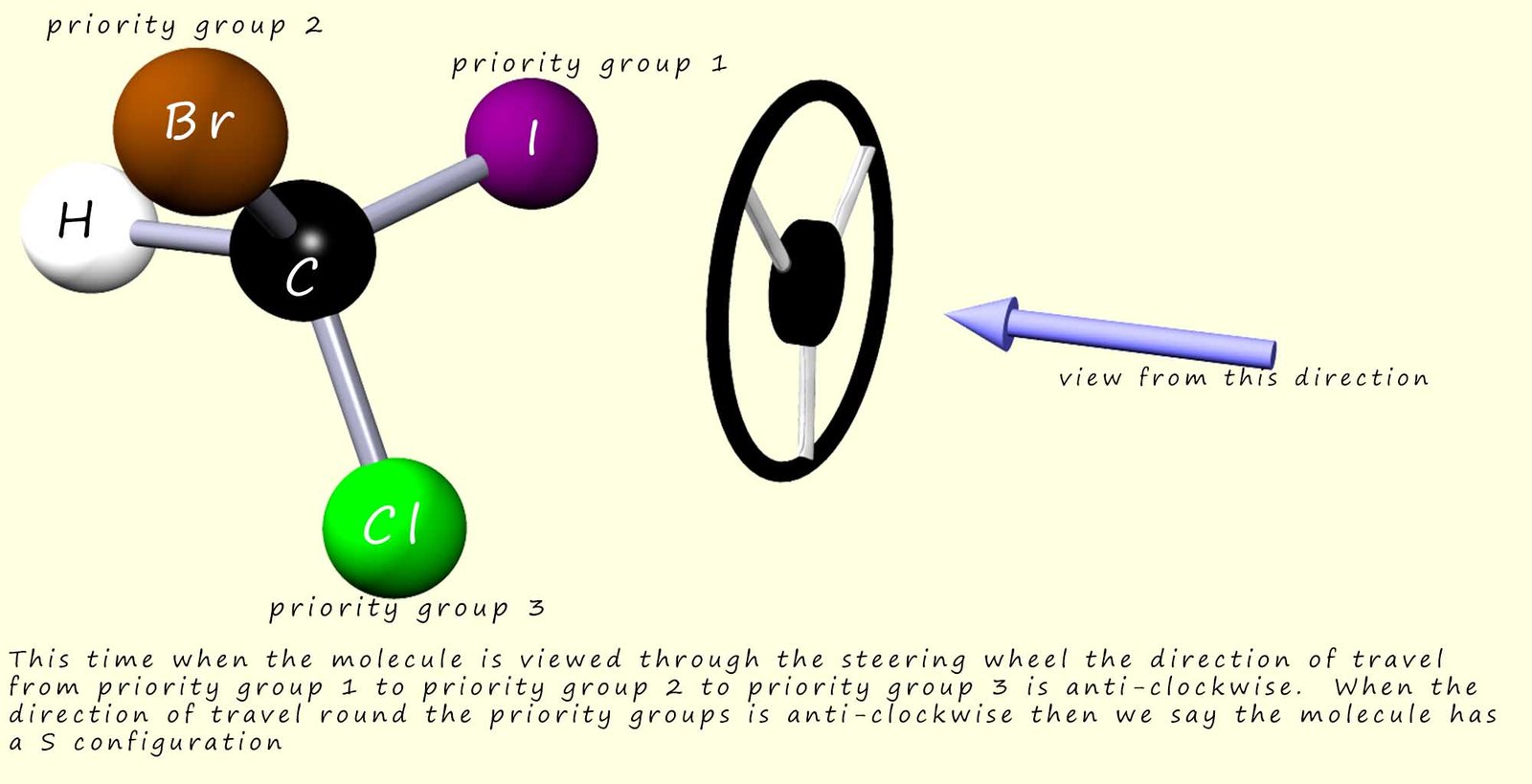
You will no doubt see molecules labelled with both the direction of rotation of plane polarised light and also with their relative configuration e.g. (R)(-)-lactic acid, here we should be able to draw the structure of (R)-lactic acid based on the method discussed above and from this work out the structure of (S)(+)-lactic acid.
It is worth noting that there is NO method which will enable you to work out whether one enantiomer of any particular molecule will be laevorotatory or dextrorotatory. The only way to know is to put a sample of the molecule in a polariser and measure the direction and size of the rotation of plane polarised light. Just because in the example I have given, namely (-)-lactic acid, the laevorotatory enantiomer has a R-configuration does not mean that all enantiomers which are laevorotatory will have a R-configuration. There is no link between the direction of rotation of plane polarised light and the relative configuration around a chiral carbon atom.