

This page is designed to give you a little more detail on the Cahn, Ingold, Prelog (CIP) sequence or priority rules. It follows on from the previous page on stereoisomers, where we looked at cis/trans isomerism in alkenes and were introduced to the E/Z naming system. Although the CIP rules were used there to help identify E and Z geometric isomers, this page focuses more carefully on how those rules work and how to apply them step by step. Before doing that, we will start with a short recap of the cis/trans and E/Z naming systems for alkenes, although I would recommend you read the page on stereoisomers if you are in any doubt.
The cis/trans naming system works well for substituted alkenes where there is an identical atom/group attached to each carbon atom in the carbon–carbon double bond (C=C). In these cases the cis geometric isomer has the identical groups on the same side of the molecule, while in the trans geometric isomer the identical groups are on opposite sides of the C=C bond. This is outlined in the image below, which shows the two geometric cis and trans isomers of 1,2-dichloroethene:
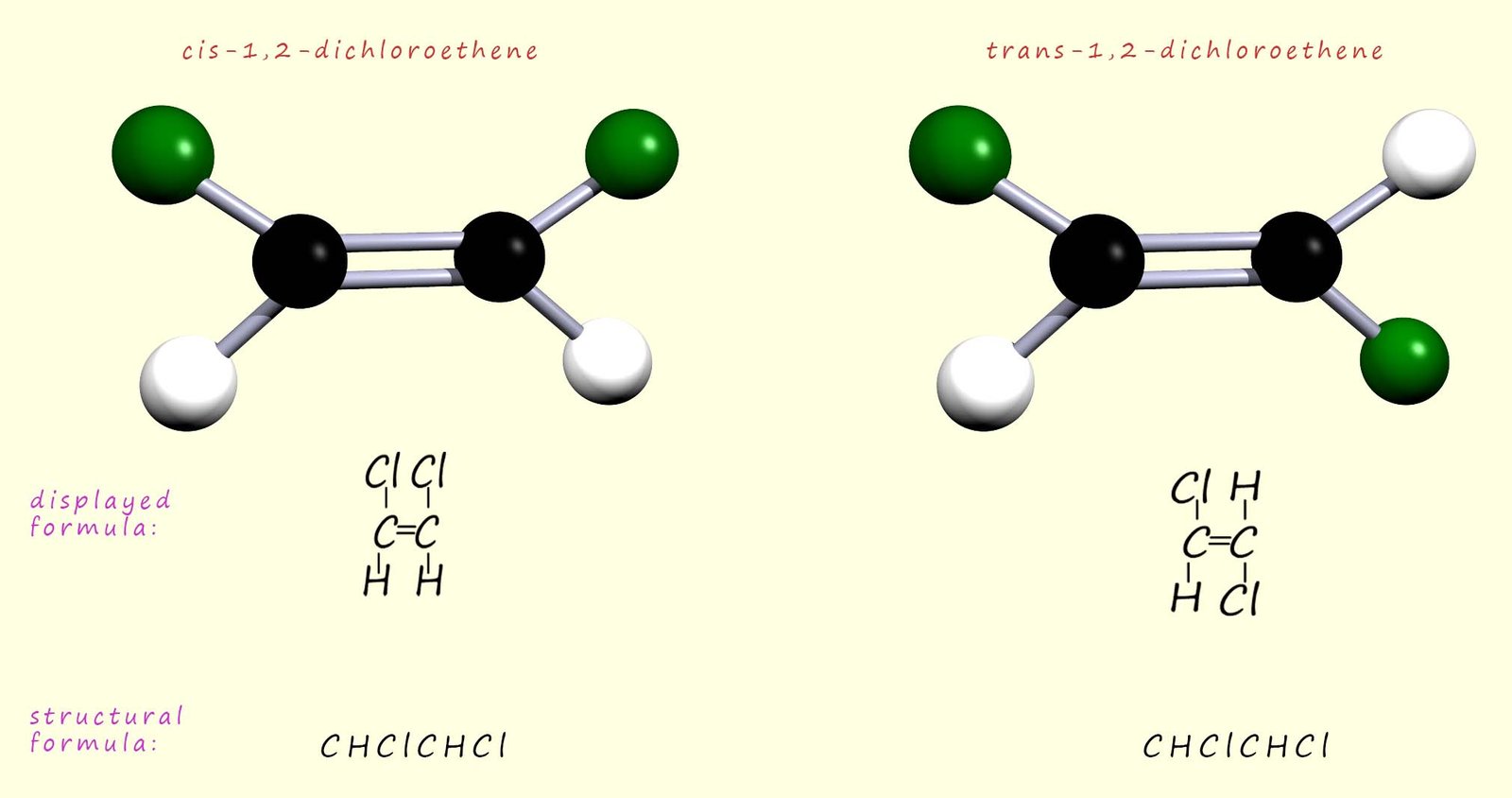
The cis/trans naming system is really handy for naming and identifying many substituted alkenes, but it only works when there is an identical group attached to each carbon of the C=C bond. Once this condition is not met, the terms cis and trans become ambiguous and can no longer be used reliably. As an example, consider the molecule shown below; here there are four different substituents in total attached to the C=C bond, so the cis/trans naming system cannot be used to name these geometric isomers.
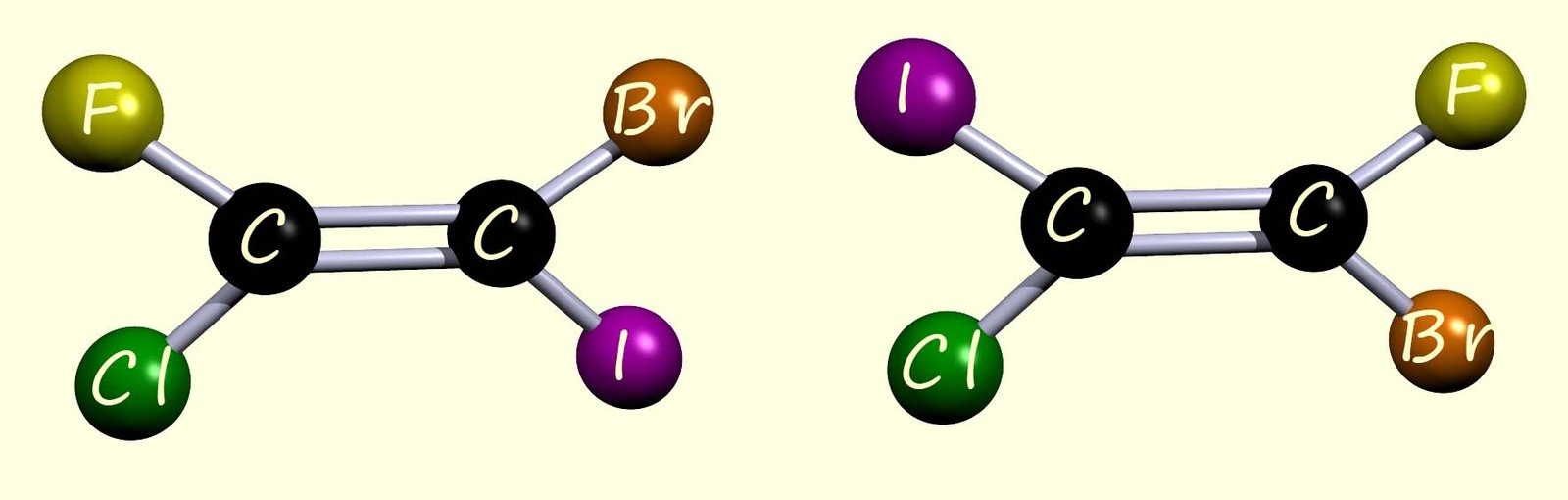
To name the two geometric isomers above we use the E/Z naming system instead. Now the E/Z naming system is based on the Cahn-Ingold-Prelog (CIP) sequence rules, which assign an order of priority to the groups attached to each carbon atom in the carbon–carbon double bond (C=C).
So for example in the two geometric isomers mentioned above, look at each atom in turn which is attached directly to each of the carbon atoms in the carbon-carbon double bond (C=C) and rank them in order of their atomic number. The higher the atomic number, the higher the priority of the substituent. This is shown in the image below:
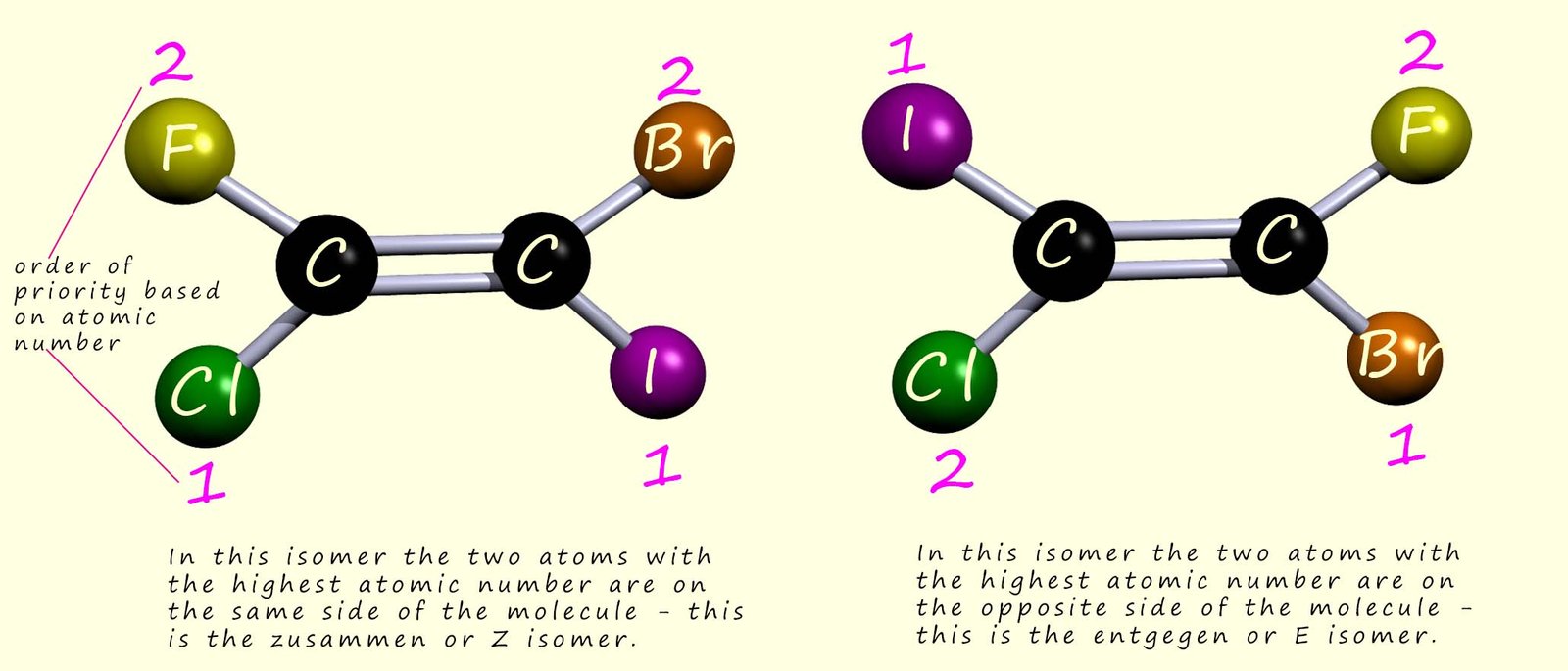
The numbers beside each atom in the image above show the order of priority based on the atomic number of each atom; the higher the atomic number of the attached atom, the higher its priority.
If the two higher priority groups are on the same side of the molecule then the molecule is designated the Z-isomer (z for zusammen, German for together), and if the two higher priority groups are on opposite sides of the molecule then this isomer is designated the E-isomer (e for entgegen, German for opposite). This is outlined in the image above.
If two isotopes of the same element are present in an alkene molecule then the isotope of higher mass takes higher priority. So, for example, deuterium (2D or 2H) has a higher priority than hydrogen (1H).
If the two atoms attached to the carbon atom in the carbon-carbon double bond (C=C) are identical then we use the atomic numbers of the next attached atoms in the chain to decide on the order of priority. All you need to do is move along the attached chain of atoms until you find a difference; at this point the atom with the highest atomic number will take priority.
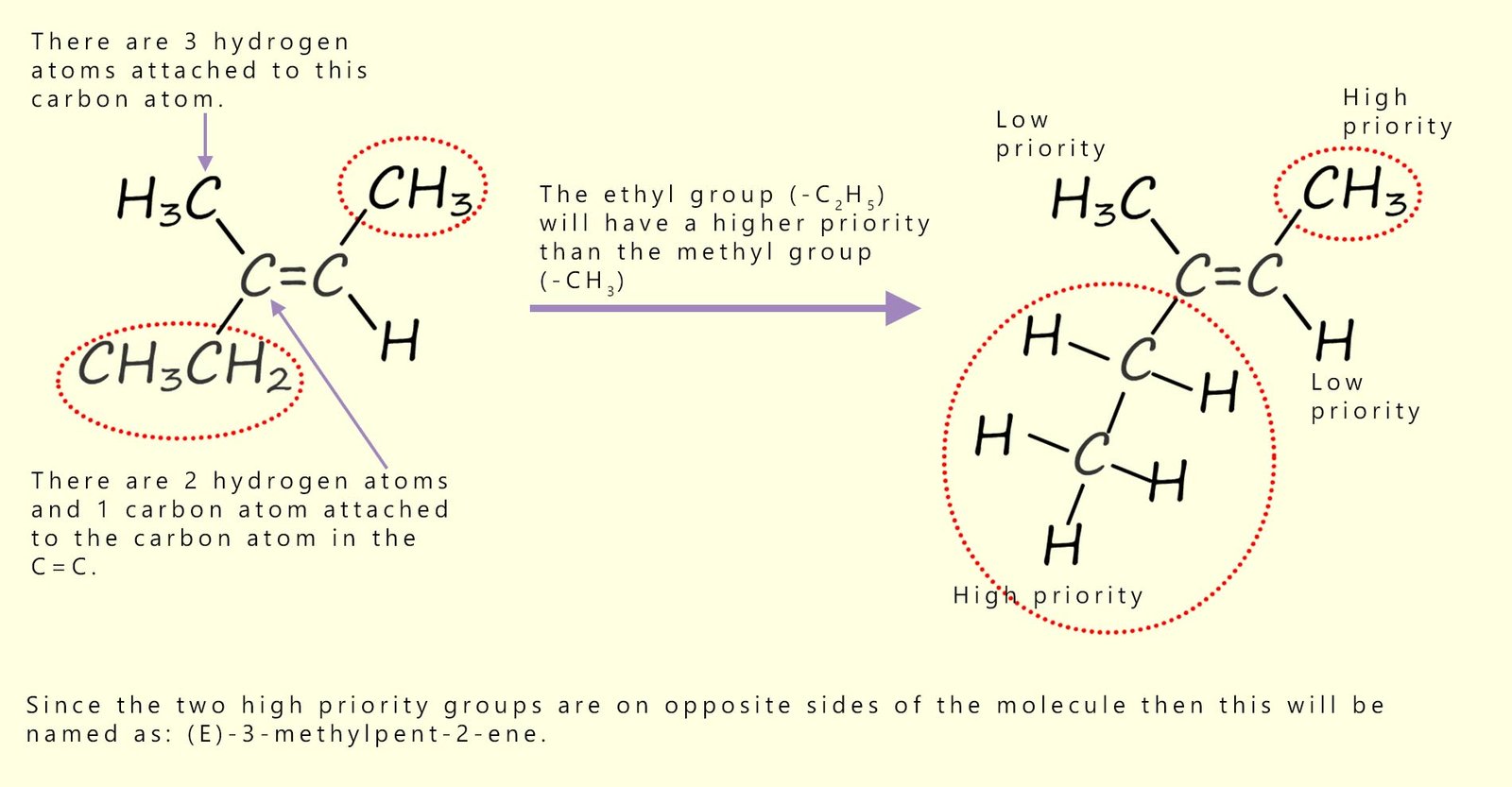
As an example consider the trisubstituted alkene molecule shown below. Here one of the carbon atoms in the carbon-carbon double bond has a methyl (-CH3) group and an ethyl group (-CH2CH3) attached. Following the above rule will result in the ethyl group having a higher priority than the methyl group.
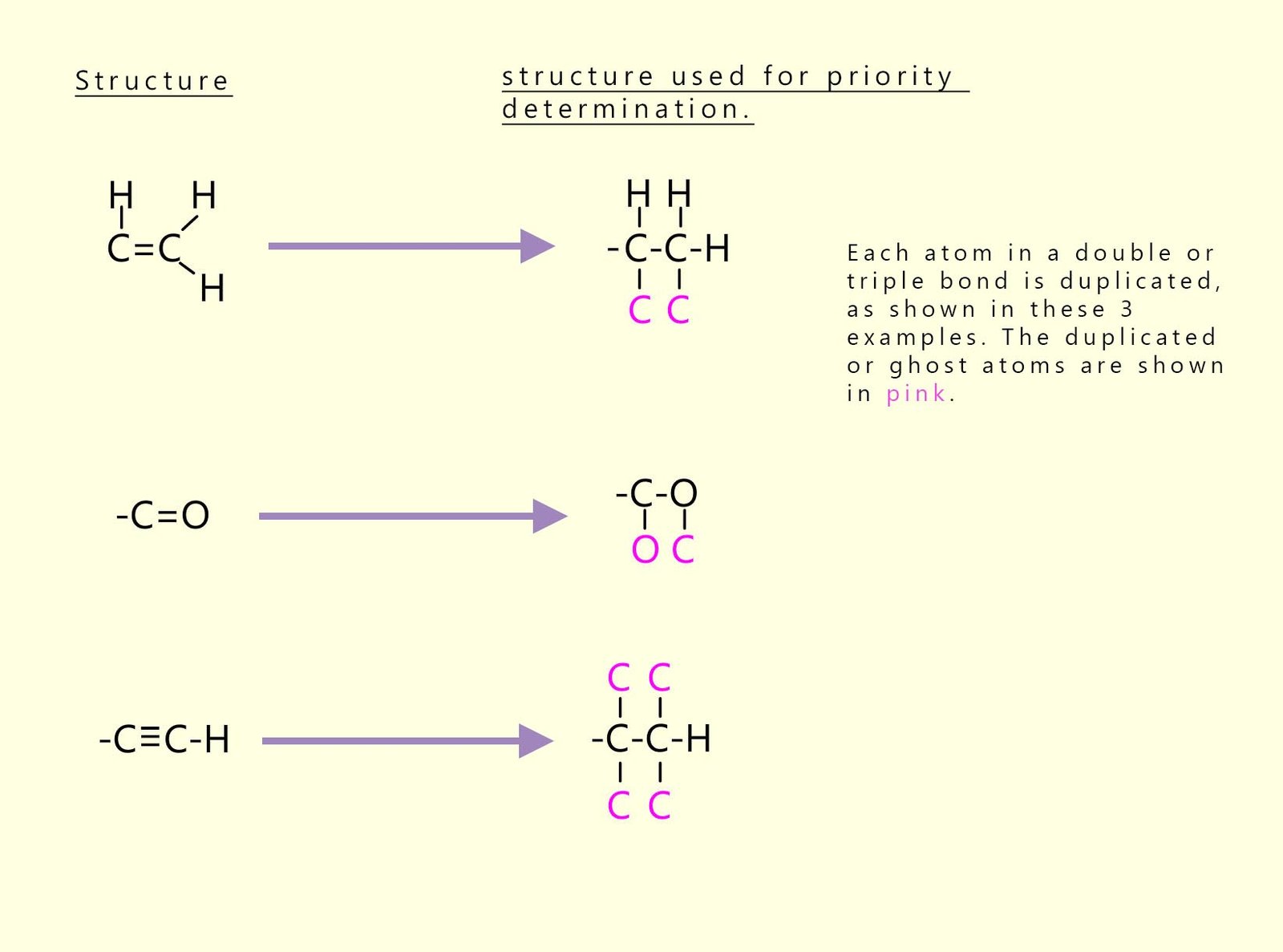
If the groups attached to the carbon-carbon double bond (C=C) contain double or triple bonds, then these groups are given a single-bond equivalency. This simply means that each double or triple bonded atom is duplicated and imaginary ghost atoms are created; for example:
Try the quick quiz before to test your understanding of the CIP sequence rules.
Pick the higher priority substituent in each pair using CIP rules, then tap Check.