

Structural formula: a formula that shows how the atoms are connected (which atoms are bonded to which).
Molecular formula: a formula that shows the number and type of each atom in a molecule (but not how they are arranged).
Stereoisomer: compounds with the same structural formula, but with atoms/groups arranged differently in 3D space.
Stereoisomers are compounds which have the same structural formula but the atoms are arranged differently in 3D space.
There are two types of stereoisomers:
On this page we will focus on geometric isomers but if you would like some information on optical isomers then click here.
The diagram below shows two molecules of 1,2-dichloroethane (C2H4Cl2). These two molecules appear to have a different shape from each other; so are they isomers? They have the same structural formula but the atoms are arranged differently in 3D space, so they appear to fit the definition for a stereoisomer!
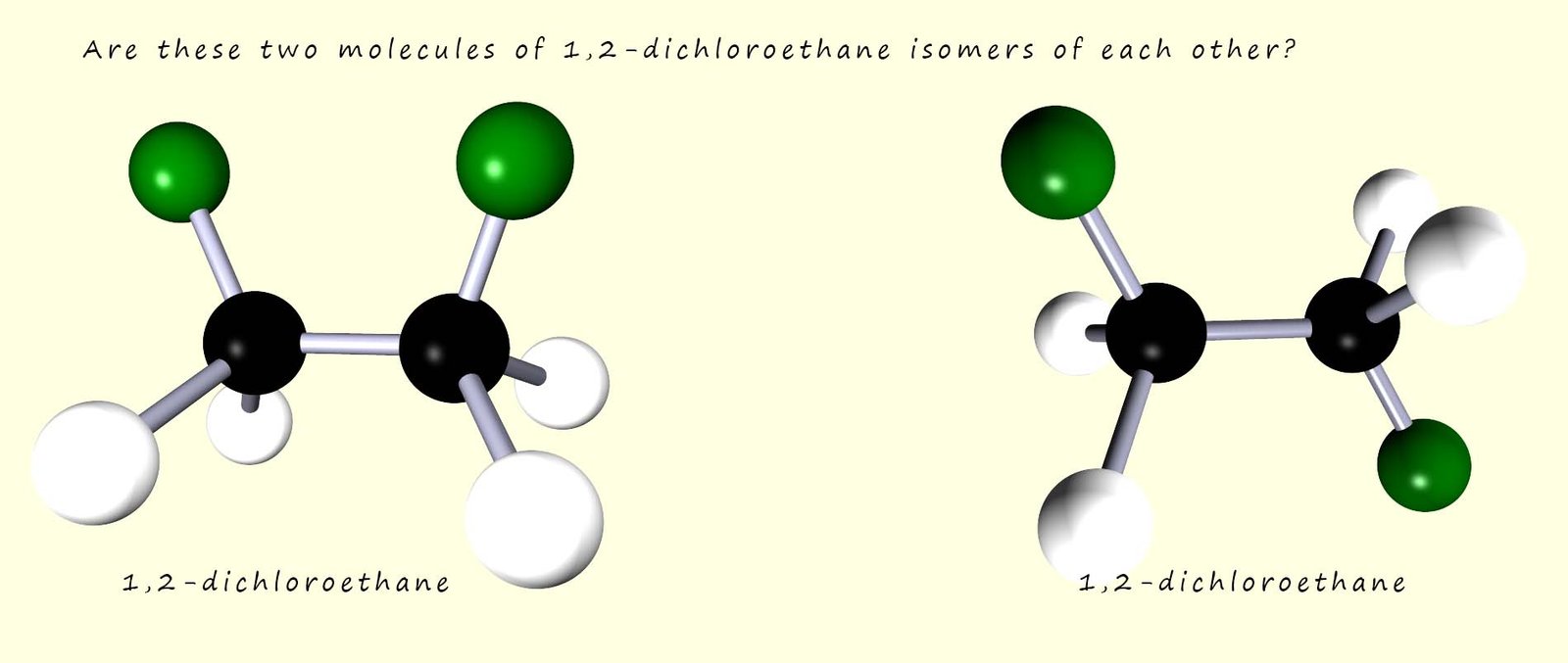
At first glance you may think that these two molecules are
indeed isomers; however they are NOT.
The reason they are not isomers is simple enough; there is free rotation
about the carbon-carbon bond.
If you can imagine that each of the carbon atoms and all the groups attached to them are in
constant rotation,
this means that these two apparent isomers are actually the same compound. It is possible to
convert one of these apparent isomers into the other by
simply rotating the carbon-carbon bond as shown below:
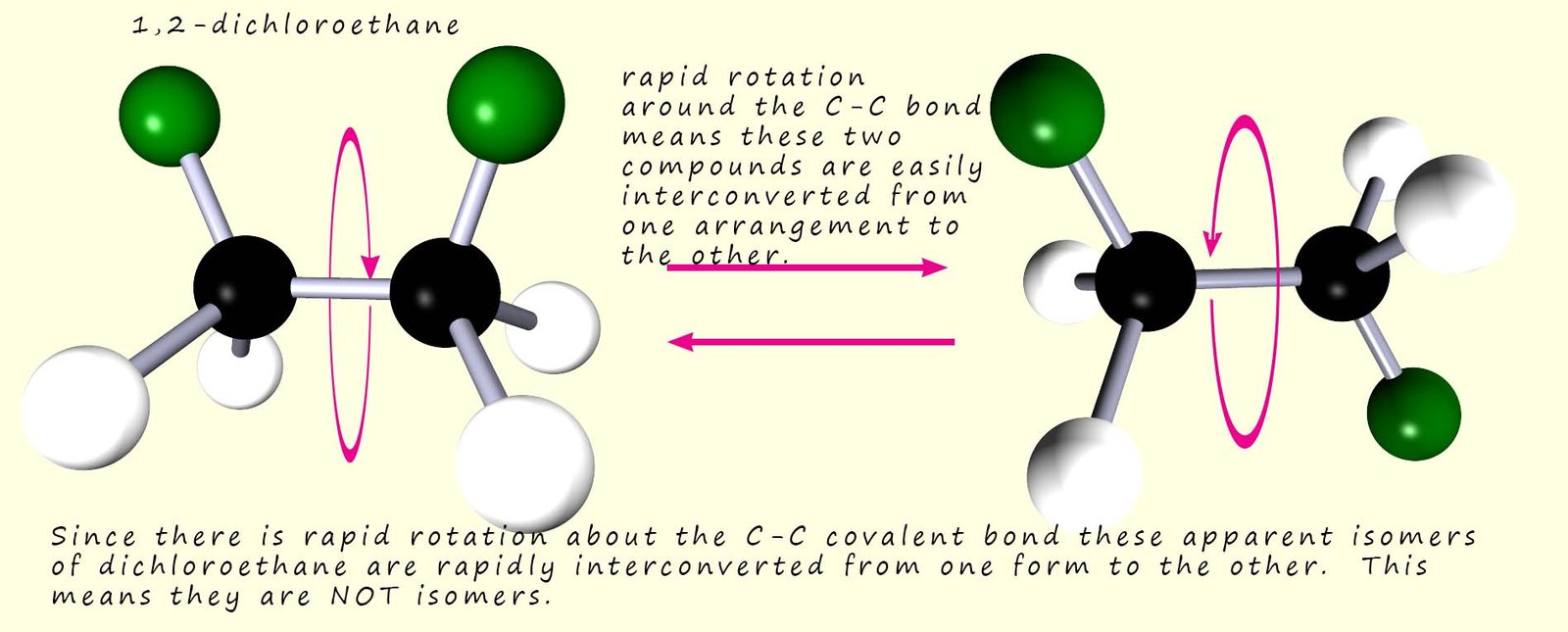
However if we could restrict or stop the rotation about the C-C bond then we would indeed have two stereoisomers. The molecules shown below are alkenes which contain the C=C functional group. The presence of the double covalent bond between the atoms of carbon stops any rotation:
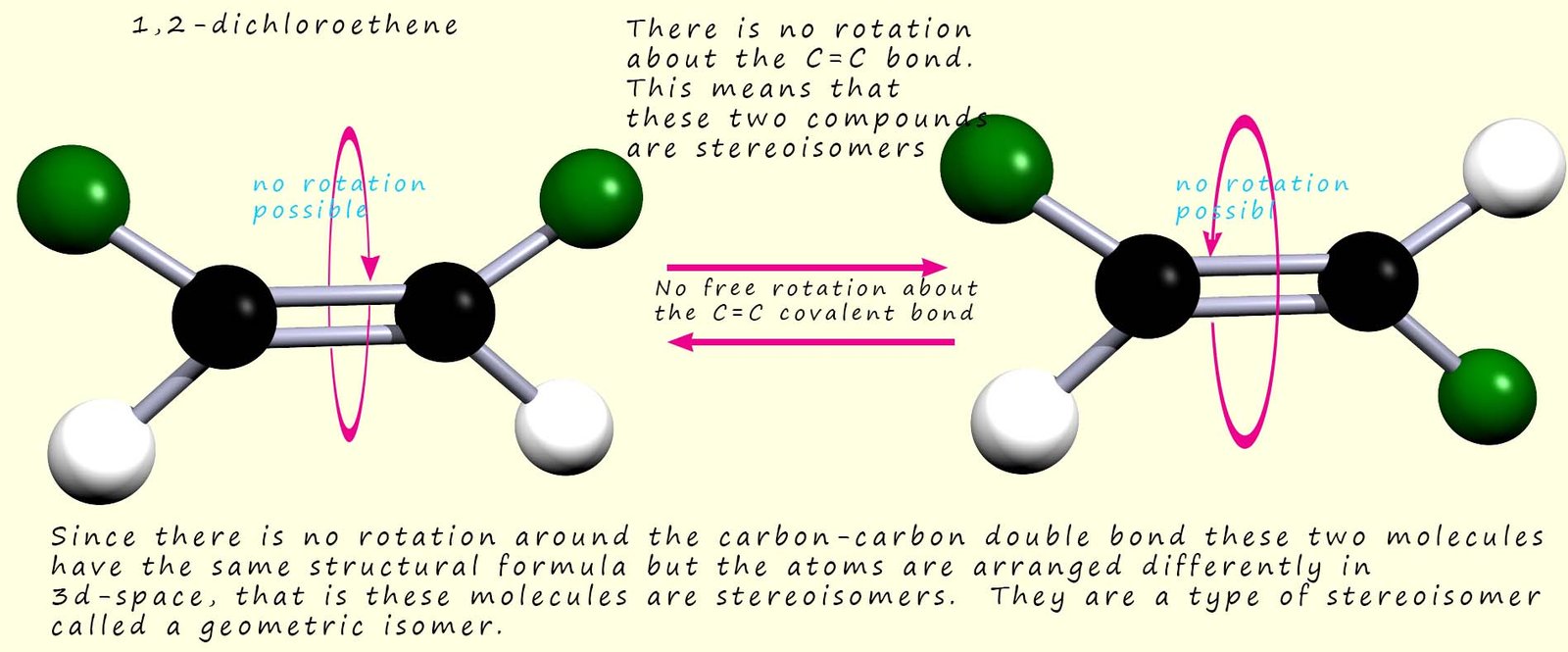
How would we name these two different geometric isomers of 1,2-dichloroethene?
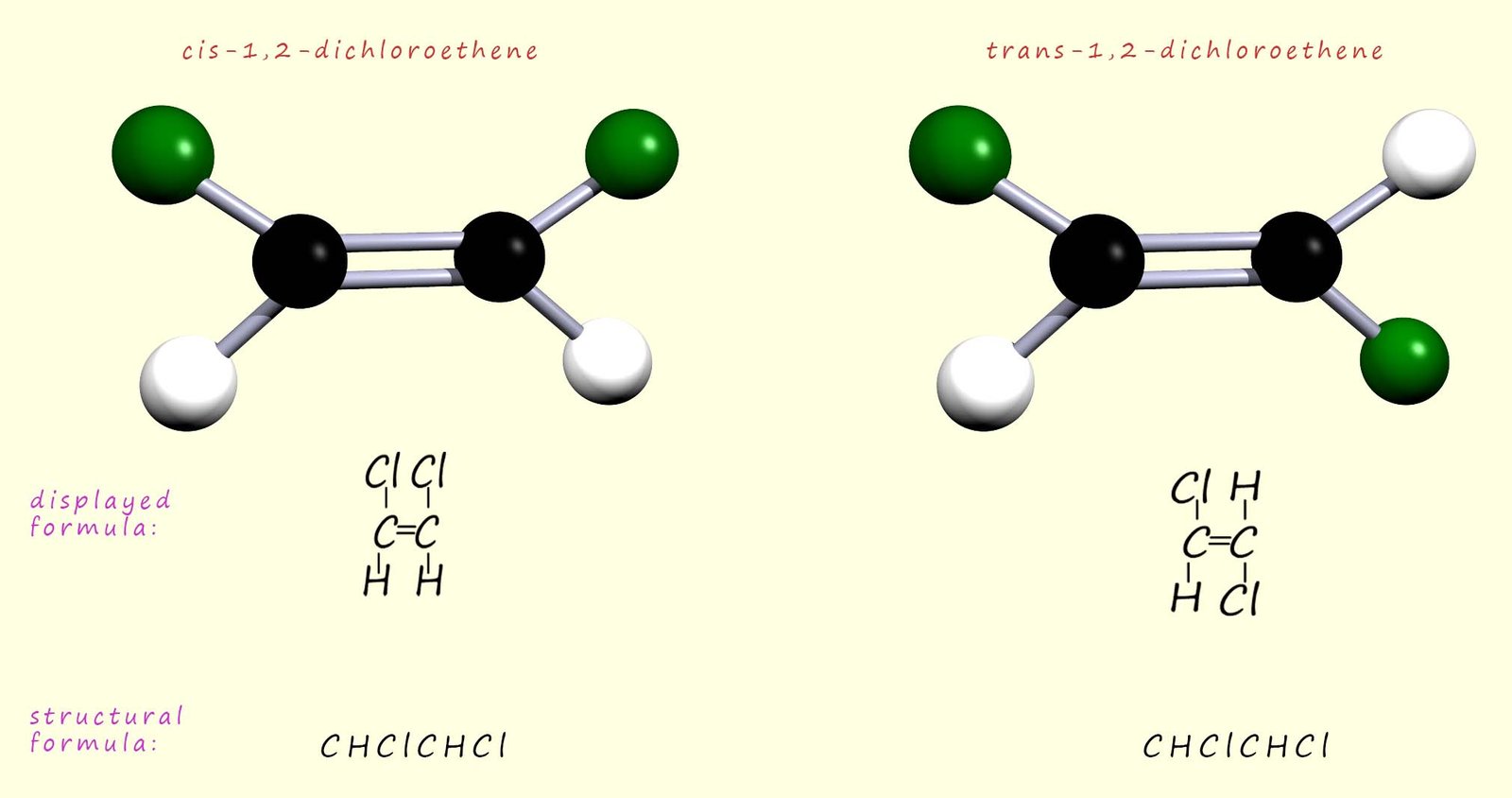
The traditional method of naming these two isomers is using the
prefixes cis and trans. In the
cis-isomer both the
chlorine atoms are on the same side of the molecule
and the hydrogen atoms are on the other, whereas in the trans-isomer
the two chlorine atoms are on opposite sides of the molecule. This is shown in the image below:
As another example of a geometric isomer consider the alkene molecule but-2-ene which exists as a pair of geometric isomers. The cis and trans isomers of but-2-ene are shown in the image below. In the cis-isomer the two methyl groups (CH3) attached to the carbon atoms in the C=C are on one side of the molecule, whereas in the trans-isomer the two methyl groups (CH3) are on opposite sides of the molecule:
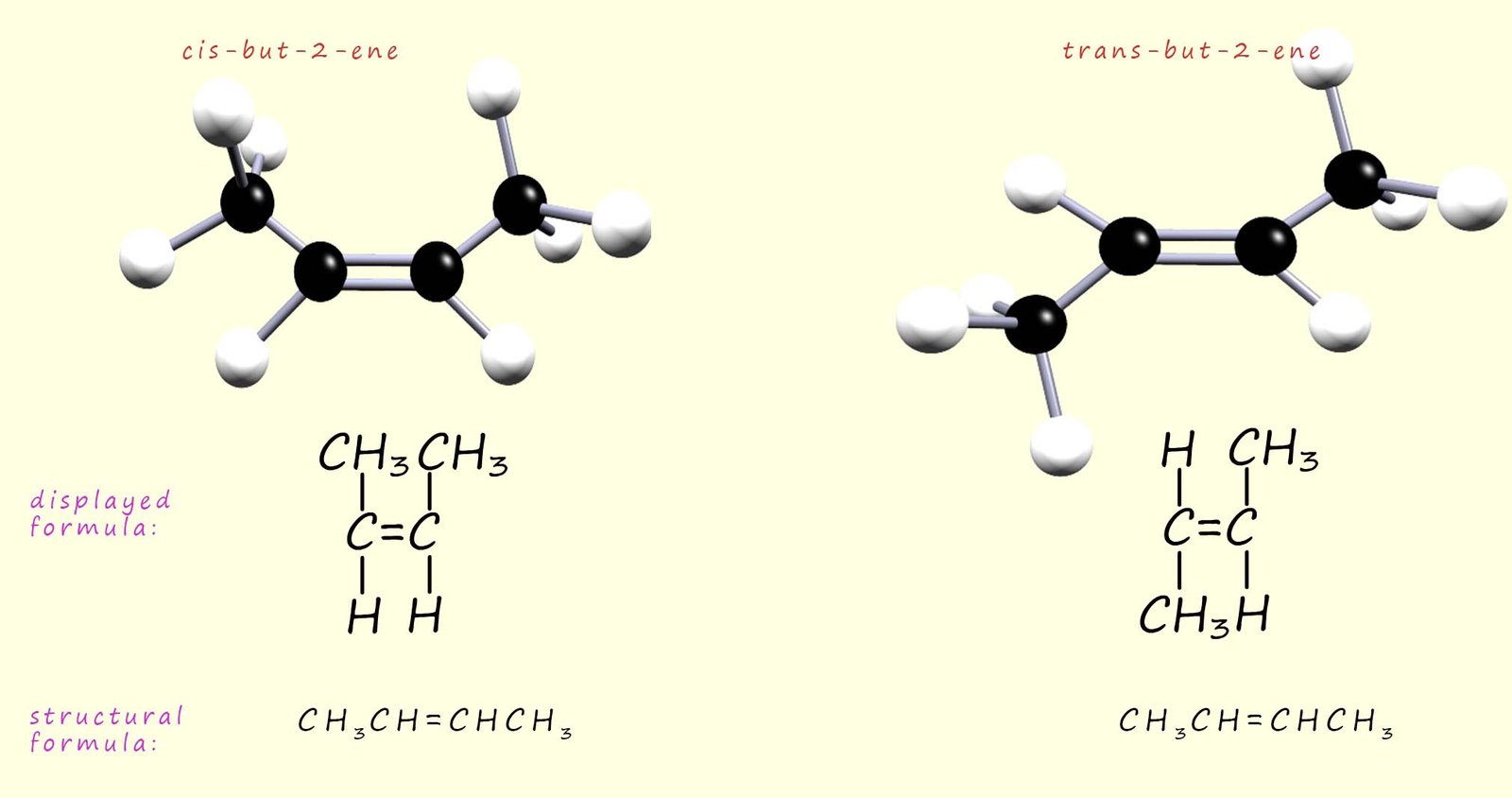
Care is needed in deciding if a particular alkene will show
geometric isomerism; that is will it have a cis and
trans geometric isomers. The
main requirement for cis/trans isomerism is that the two groups
bonded to each of the carbon atoms in the carbon-carbon
double bond (C=C) are different. The cis/trans naming system is used to
name disubstituted alkenes.
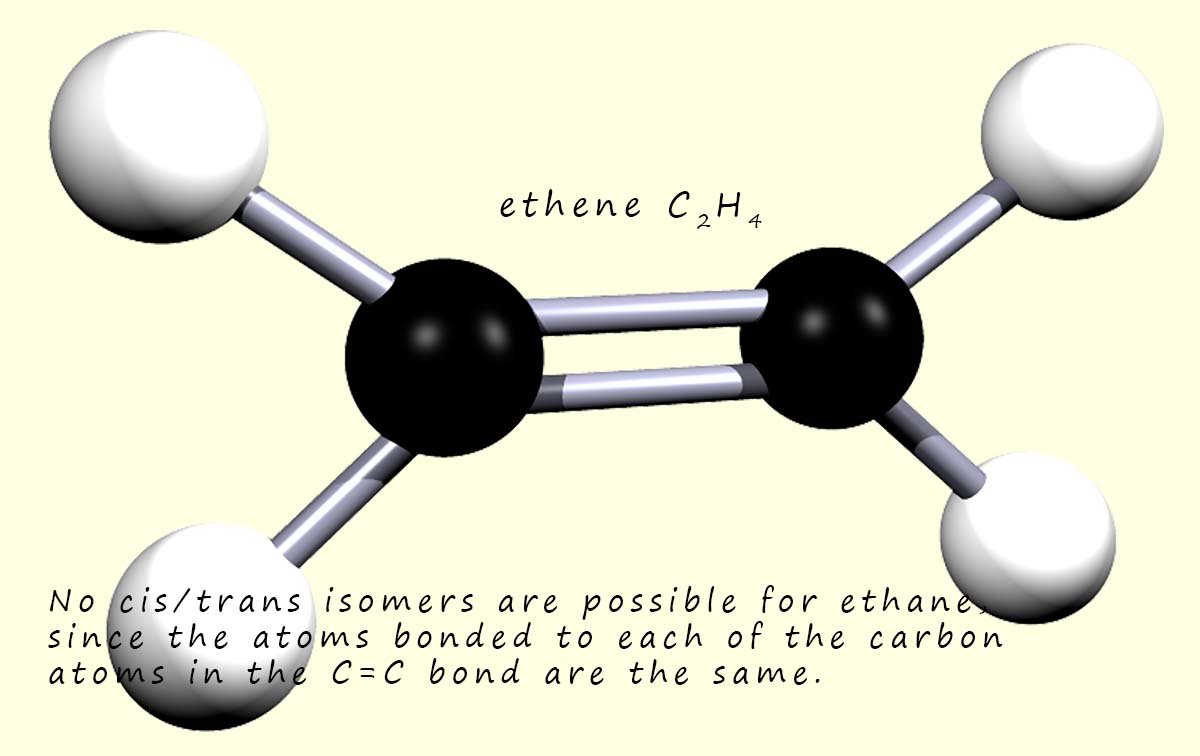
So the alkene ethene shown opposite will not have cis/trans geometric isomers since
each of the carbon atoms in the carbon-carbon double bond is bonded
to identical hydrogen atoms.
In the example above, but-2-ene can exist as a pair of geometric isomers, however but-1-ene does not have any cis/trans geometric isomers. The reason for this is the same reason why ethene has no geometric isomer: two of the atoms attached to one of the carbon atoms in the C=C are the same. This is shown in the image below:
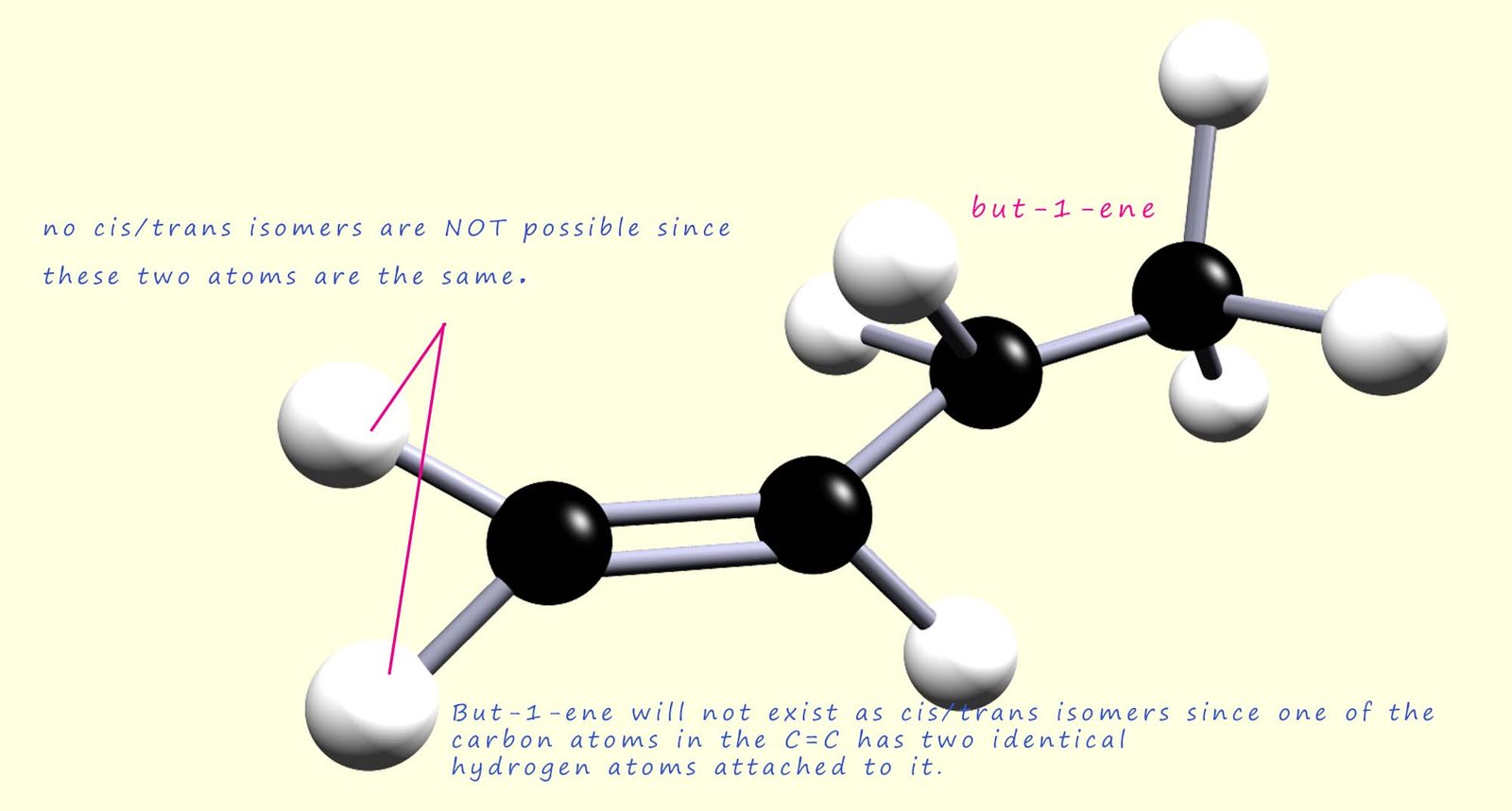
In the examples used above to illustrate cis-trans isomerism we used alkenes that were disubstituted with two different groups on each carbon atom in the C=C bond, but in each case two of the groups on each carbon atom were the same. The cis-trans naming system is useful for describing and naming disubstituted alkenes; however, it has some limitations. For example; the two molecules shown below are stereoisomers and specifically geometric isomers, however it would prove impossible to identify which is the cis and which is the trans isomer based on the information provided above. These alkene molecules cannot be named using the cis/trans naming scheme because there is no identical group attached to each carbon in the C=C bond, so how do we name these geometric isomers if we cannot use the cis/trans naming scheme?
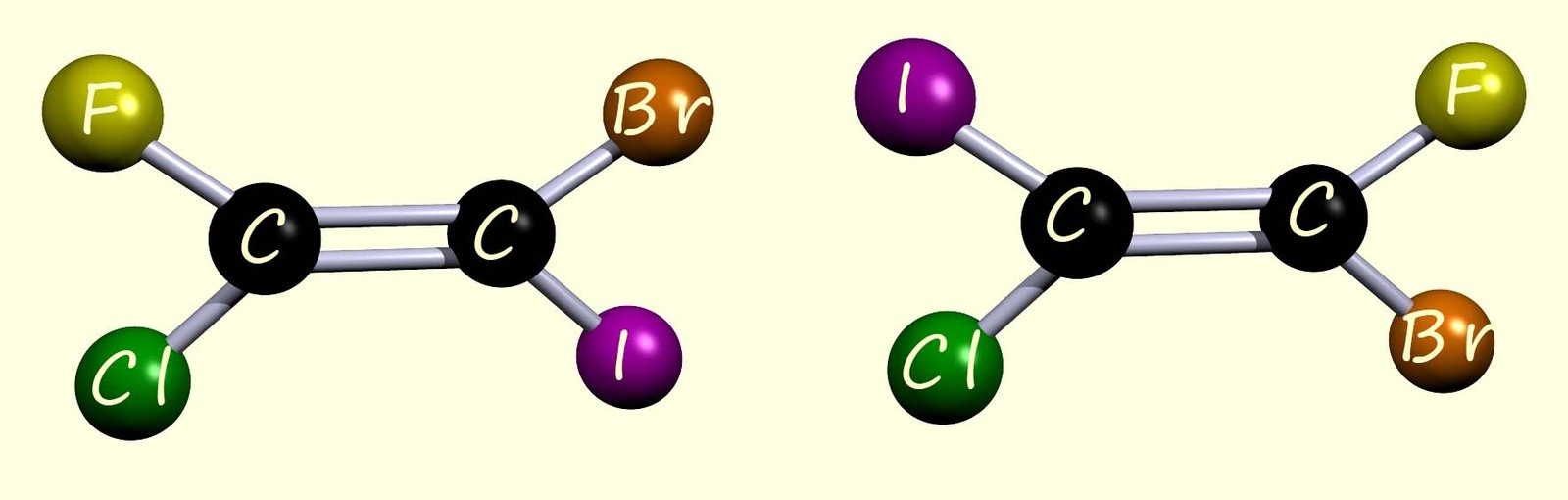
An improved naming system called the E-Z system can be used to describe these two
geometric isomers. This system
uses the atomic number (more precisely, it uses the Cahn-Ingold-Prelog rules)
to put the groups in order of priority. So for the two geometric isomers
mentioned above, the numbers beside the atoms in the image below
show the order of priority based on the atomic number of each atom,
the higher the atomic number of the attached atom; the higher
its priority.
If the two higher priority groups are on the same side
of the molecule then the molecule is designated the Z-isomer
(z for zusammen, German for together), and if the two higher priority groups
are on opposite sides of the molecule then
this isomer is designated the E-isomer
(e for entgegen, German for opposite). This is outlined in the image below:
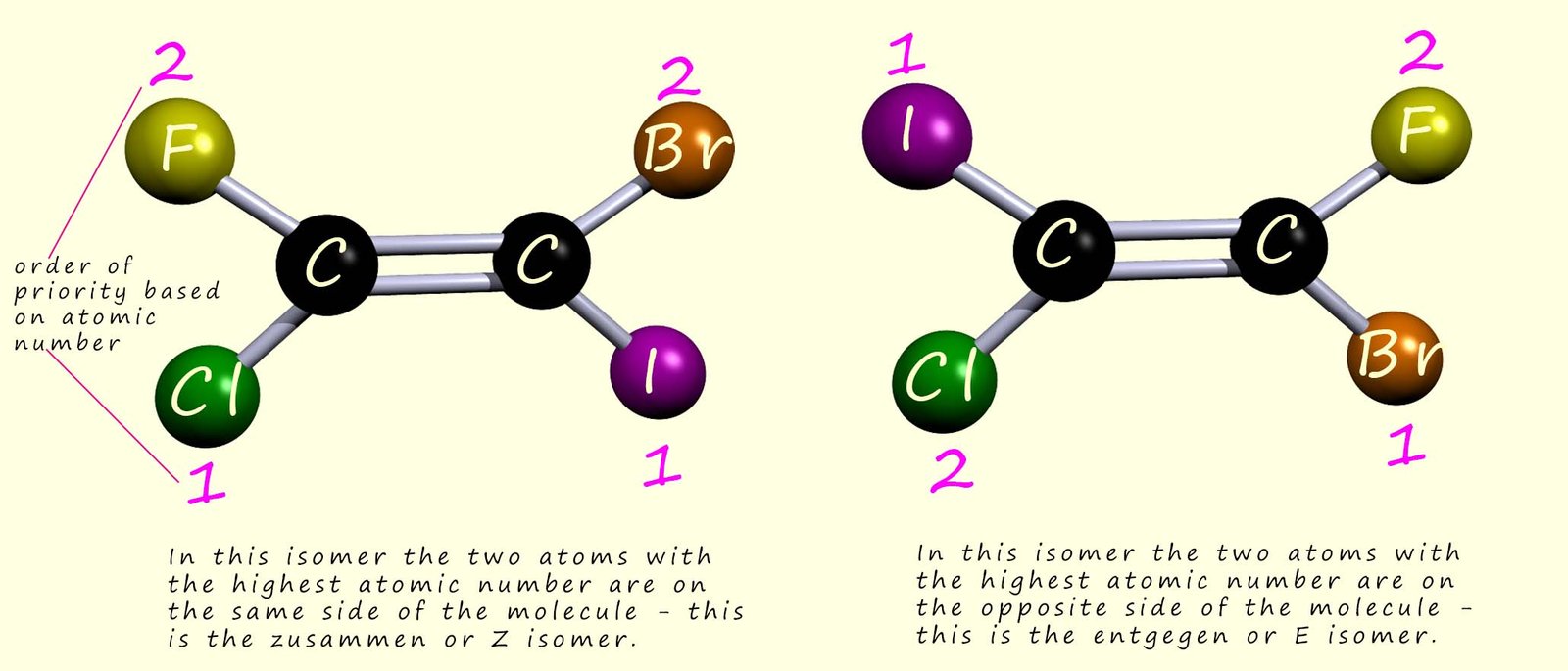
So although the cis-trans naming system does not work for all
molecules, it is still widely used in chemistry to name
disubstituted alkenes and you should not dismiss it
out of hand. You will meet lots of molecules which are described using this system.
However, the E-Z system has the advantage over the cis-trans
system in that it works for all molecules.
However, it is clearly possible to use both systems to describe simple molecules;
the image below shows the cis-trans isomers of but-2-ene which we met earlier; but they
are also identified using the E-Z system where
the order of priority of the atoms/groups attached to the carbon atoms in the
C=C are labelled.
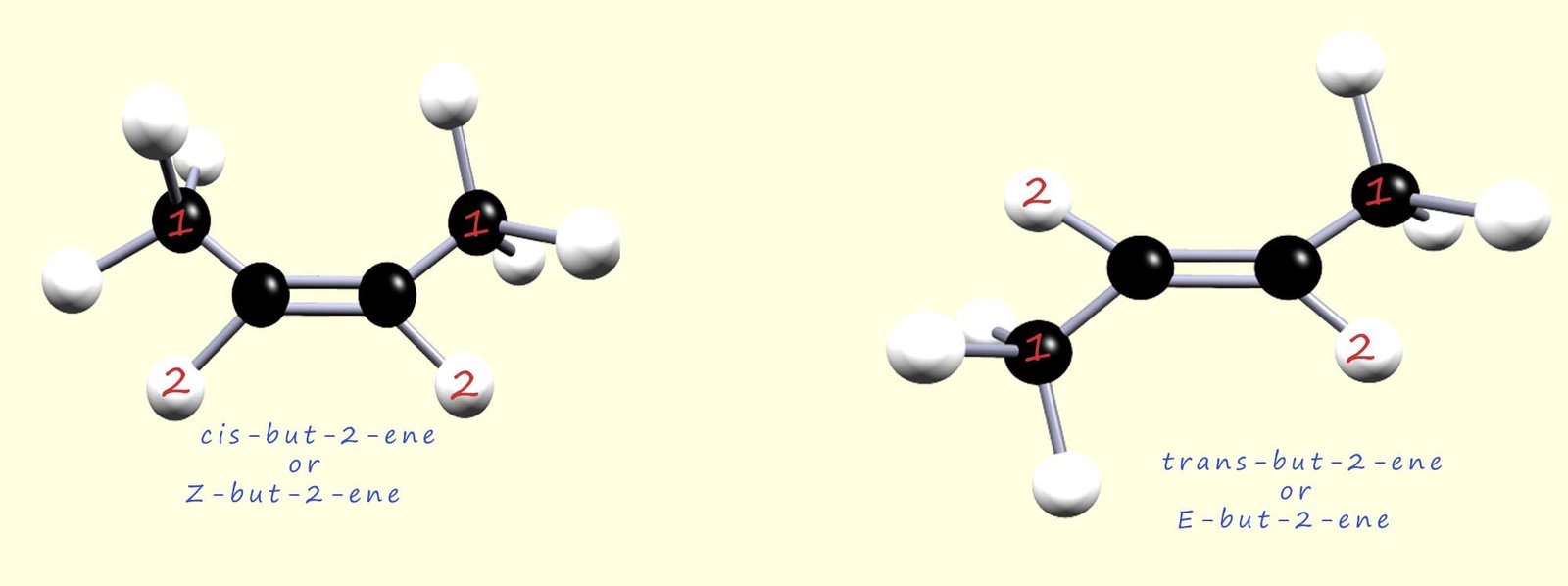
| Concept | What it shows | When it applies | Key exam point |
|---|---|---|---|
| Structural formula | How atoms are connected | When comparing isomers | Stereoisomers must have the same structural formula |
| Molecular formula | Number and type of atoms | All compounds | Different structures can share the same molecular formula |
| Cis / trans | Same or opposite sides of a C=C bond | Simple disubstituted alkenes | An identical group must be attached to each carbon of the C=C bond |
| E / Z | Relative positions of higher-priority groups | Alkenes with restricted rotation | Uses atomic number to assign priority (CIP rules) |
| No stereoisomerism | No fixed arrangement in space | Alkanes, ethene, but-1-ene | Free rotation or identical groups prevent stereoisomerism |
Try the quick quiz below to test your understanding of stereoisomers and geometric isomers.
Try the questions first, then open the answers underneath each one.
1) Which statement best defines a stereoisomer?
Answer: B
Stereoisomers have the same structural formula, but differ in the arrangement of atoms/groups in 3D space.
2) Why are the two “different shapes” of 1,2-dichloroethane not stereoisomers?
Answer: C
Rotation about a C-C single bond interconverts the shapes, so they are the same compound, not stereoisomers.
3) Which alkene can show cis/trans isomerism?
Answer: C
For cis/trans, each carbon of the C=C must have two different groups attached. But-2-ene meets this condition.
4) Why can’t cis/trans be used for some alkenes, even when there is a C=C bond?
Answer: B
Cis/trans needs a clear pair of identical groups to compare across the double bond. When that’s not possible, use E/Z.
5) In E/Z naming, how do you decide which group has higher priority on each carbon of the C=C bond?
Answer: B
CIP priority is assigned using atomic number (and then the next atoms out if there is a tie).
6) What does Z mean in the E/Z system?
Answer: B
Z (zusammen) means the higher priority groups are on the same side of the double bond.
Try the quick quiz below to test your understanding of when to use the cis/trans or the E/Z naming systems.
Choose which naming system you should use for each alkene. Then tap Check answers.