

Covalent bonds can be considered as either sigma (σ) or pi (π) bonds. The difference between these two types of covalent bonds is due to the way in which the atomic orbitals on each atom involved in forming the covalent bond overlap with each other during bond formation.
As an example, consider what happens when two hydrogen atoms combine to form a molecule of hydrogen (H2). Hydrogen atoms contain a single electron and have an electronic configuration of 1s1; so when two hydrogen atoms react, a covalent bond forms between the two 1s orbitals which contain the electrons. Recall that a covalent bond simply involves sharing a pair of electrons. These 1s orbitals will overlap with each other and form a covalent bond between the two atoms. When the two 1s orbitals overlap or merge with each other they will form new molecular orbitals, which will contain the 2 electrons present in the hydrogen molecule. This is outlined below:
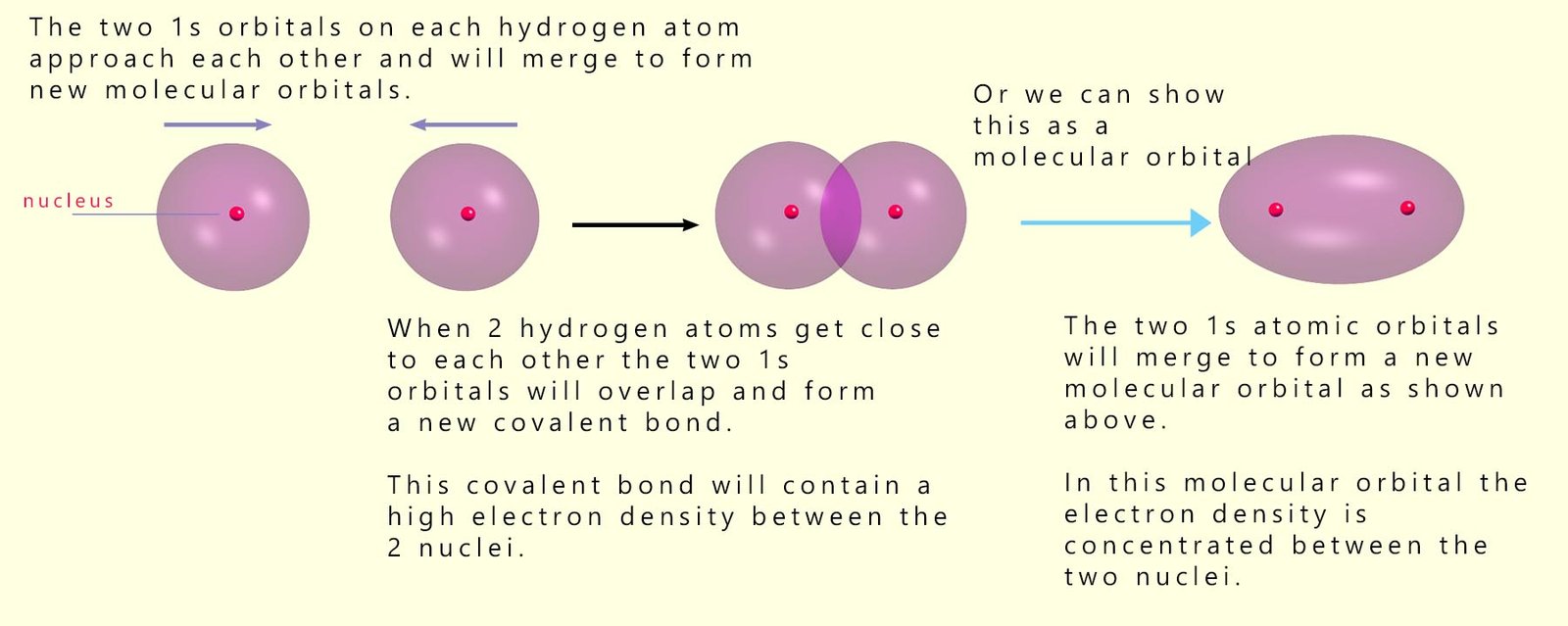
As the two hydrogen atoms approach each other, the overlap of the 1s orbitals increases. This results in a stronger bond. However, as the distance between the two nuclei decreases, the amount of electrostatic repulsion between the two positively charged nuclei will increase. A point is therefore reached where there is a balance between the attraction of the nuclei for the shared pair of electrons and the repulsion between the two nuclei. The covalent bond formed between the two hydrogen atoms is formed by the head-on overlap of the 1s orbital. Covalent bonds which are formed by this head-on overlap of atomic orbitals are also called sigma (σ) bonds.
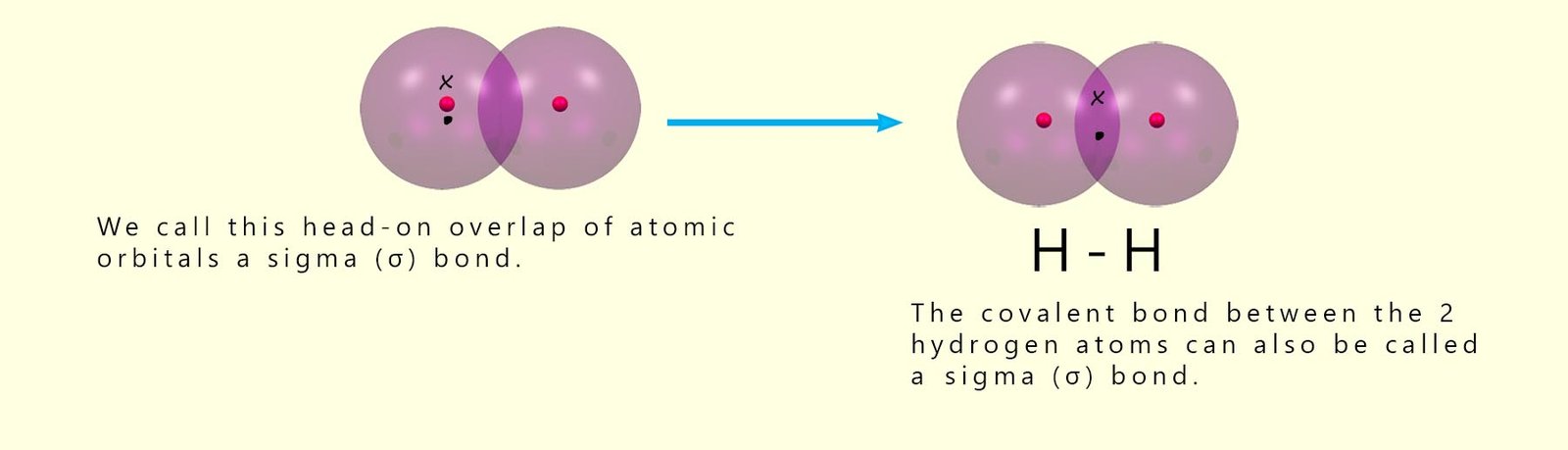
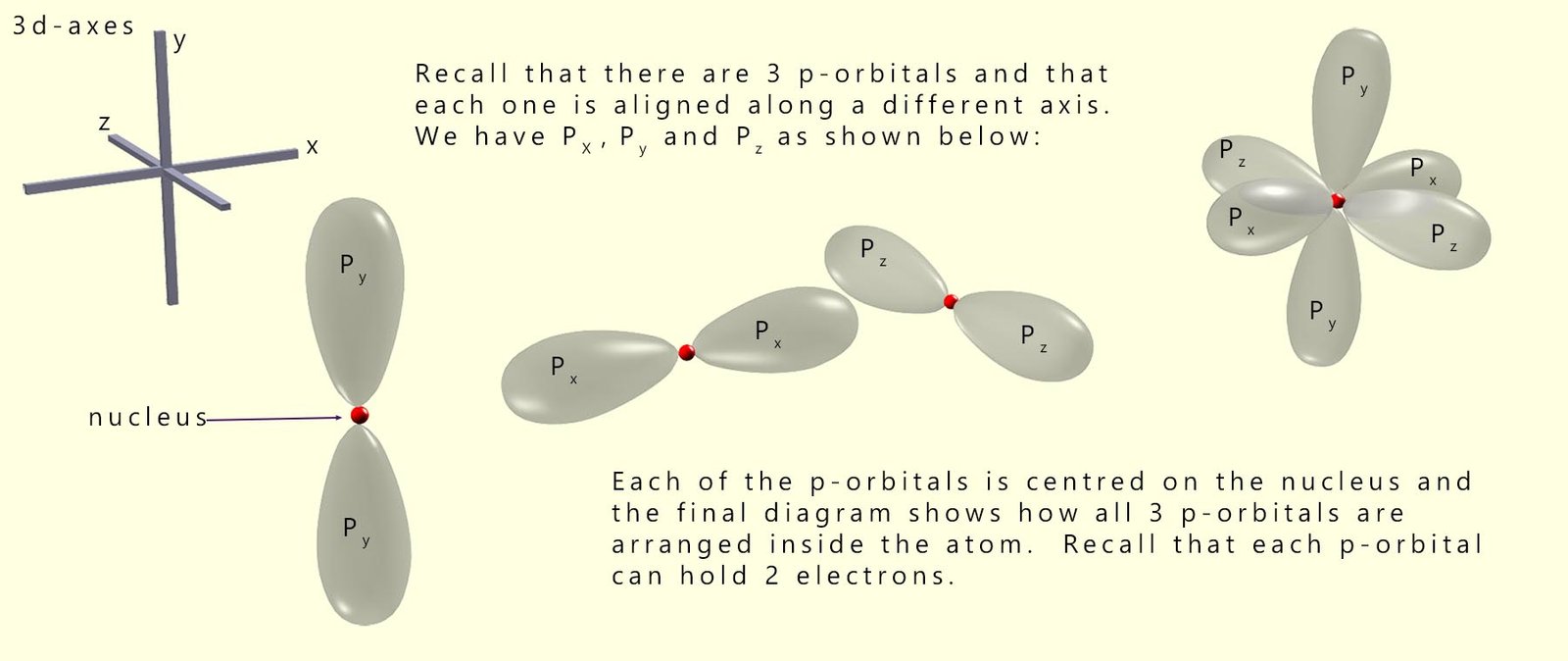
When a hydrogen molecule is formed a covalent bond forms between the 2 hydrogen atoms by the head-on overlap of two 1s-orbitals. This results in the formation of a sigma bond. However sigma bonds can also form when atomic orbitals other than s-orbitals overlap. p-orbitals can also overlap in a head-on manner to form sigma bonds. Now recall that there are three p-orbitals; px, py and pz with each orbital arranged along each of the x, y and z-axes as shown below:
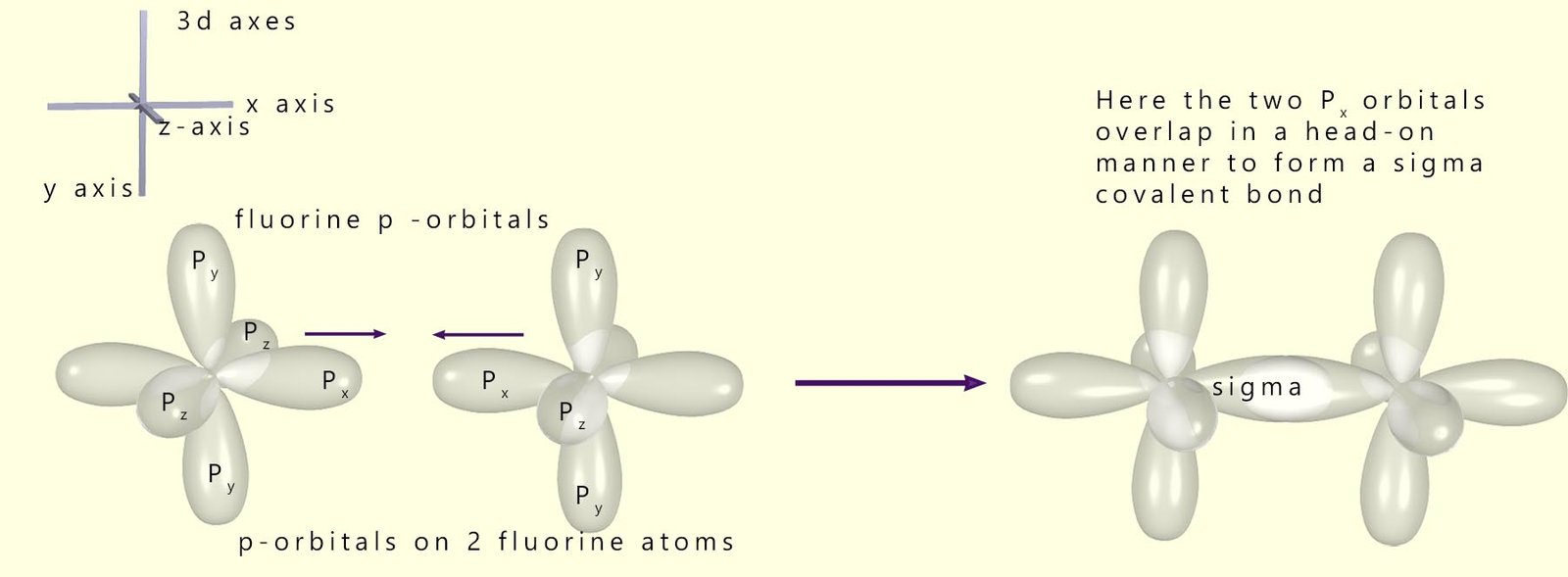
Fluorine is a halogen in group 7 of the periodic table. It has an atomic number of 9 and an electronic configuration of 1s22s22p5 or 1s22s22pz22py22px1. There are 5 electrons in the outer valence 2p sub-level, so fluorine is able to make one covalent bond in order to fill its outer energy level and end up with the same electron configuration as the noble gas neon (2s22p6).
Fluorine gas (F2), like hydrogen gas, consists of a diatomic molecule with a single covalent bond between the fluorine atoms. To completely fill the outer 2p sub-level each fluorine atom will form a covalent bond and so share a pair of electrons by a head-on overlap using one of its p-orbitals which contains the one unpaired electron; that is, it will form a covalent sigma bond by overlapping these two atomic p-orbitals. This is shown in the image below:
As we have seen above the p-orbitals in an atom can overlap in a head-on manner to form a sigma bond. However there is another
way in which the p-orbitals can overlap to form a covalent bond. Instead of the p-orbitals overlapping in a
head-on manner,
they can overlap in a sideways- manner; this sideways-overlap results in the formation of a new type of covalent bond called a
pi (π) bond. This is shown below:
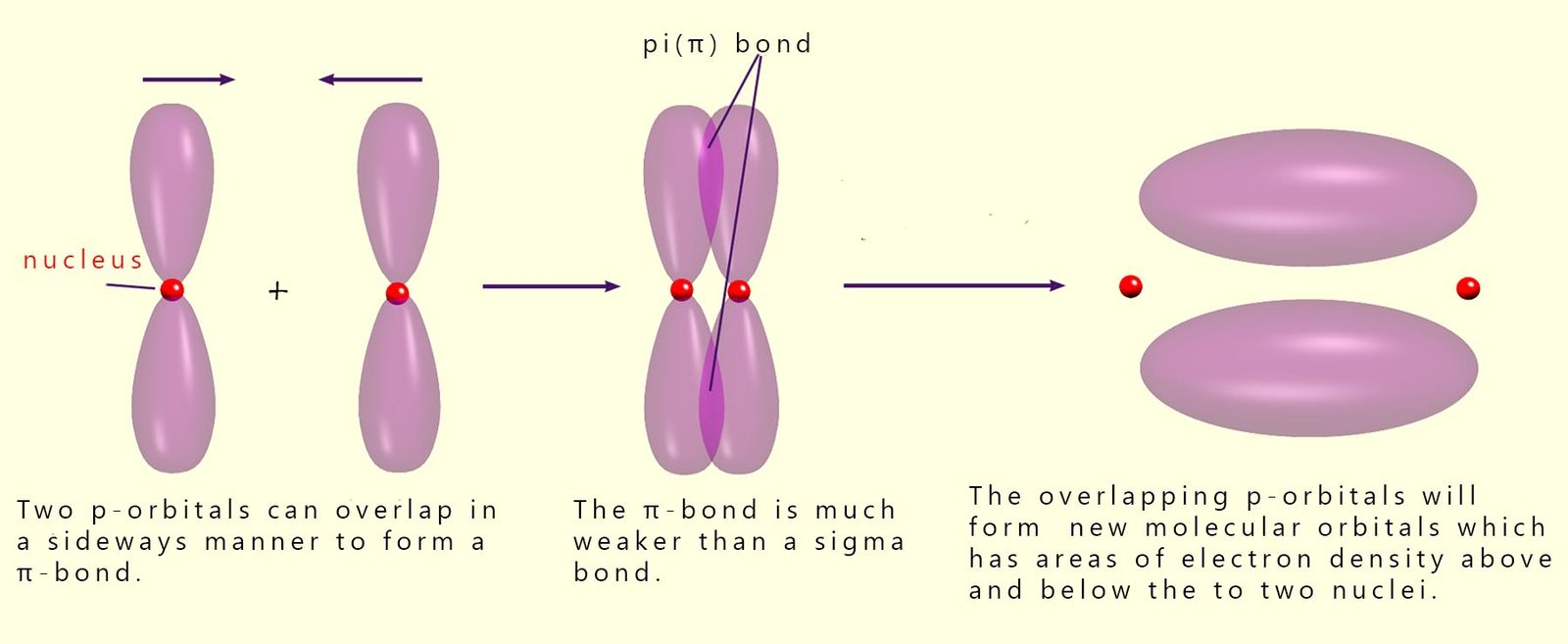 A pi (π) bond has a smaller area
of overlap between the p-orbitals than a
sigma bond, which means it is generally
a weaker covalent bond. In many molecules with double
and even triple bonds you are likely to find that the covalent bonds
between the atoms in the molecule will contain a mixture of one sigma
bond and one or more pi bonds.
A pi (π) bond has a smaller area
of overlap between the p-orbitals than a
sigma bond, which means it is generally
a weaker covalent bond. In many molecules with double
and even triple bonds you are likely to find that the covalent bonds
between the atoms in the molecule will contain a mixture of one sigma
bond and one or more pi bonds.
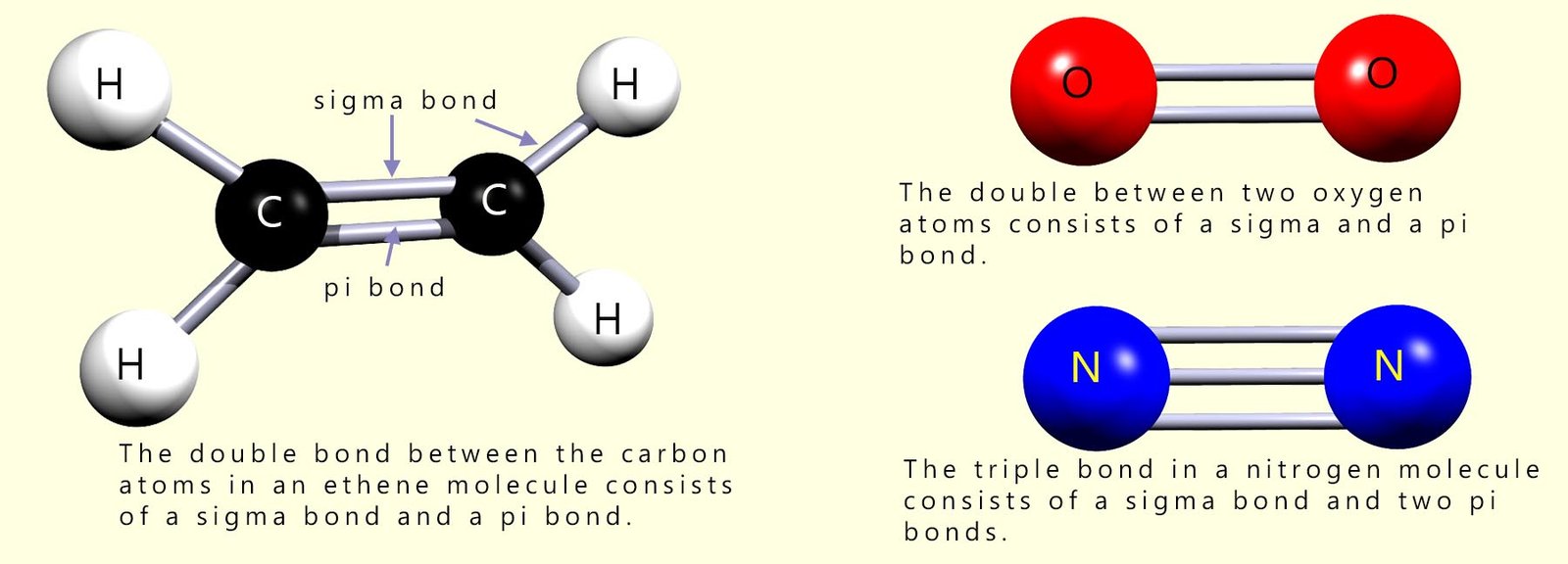
The bonding present in molecules with single covalent bonds is likely to be a sigma bond, where there is full head-on overlap of the atomic orbitals. However in molecules which have multiple bonds such as double or triple bonds then the covalent bonds between the atoms in the molecule will be a mixture of sigma and pi(π) bonds. This is shown below:
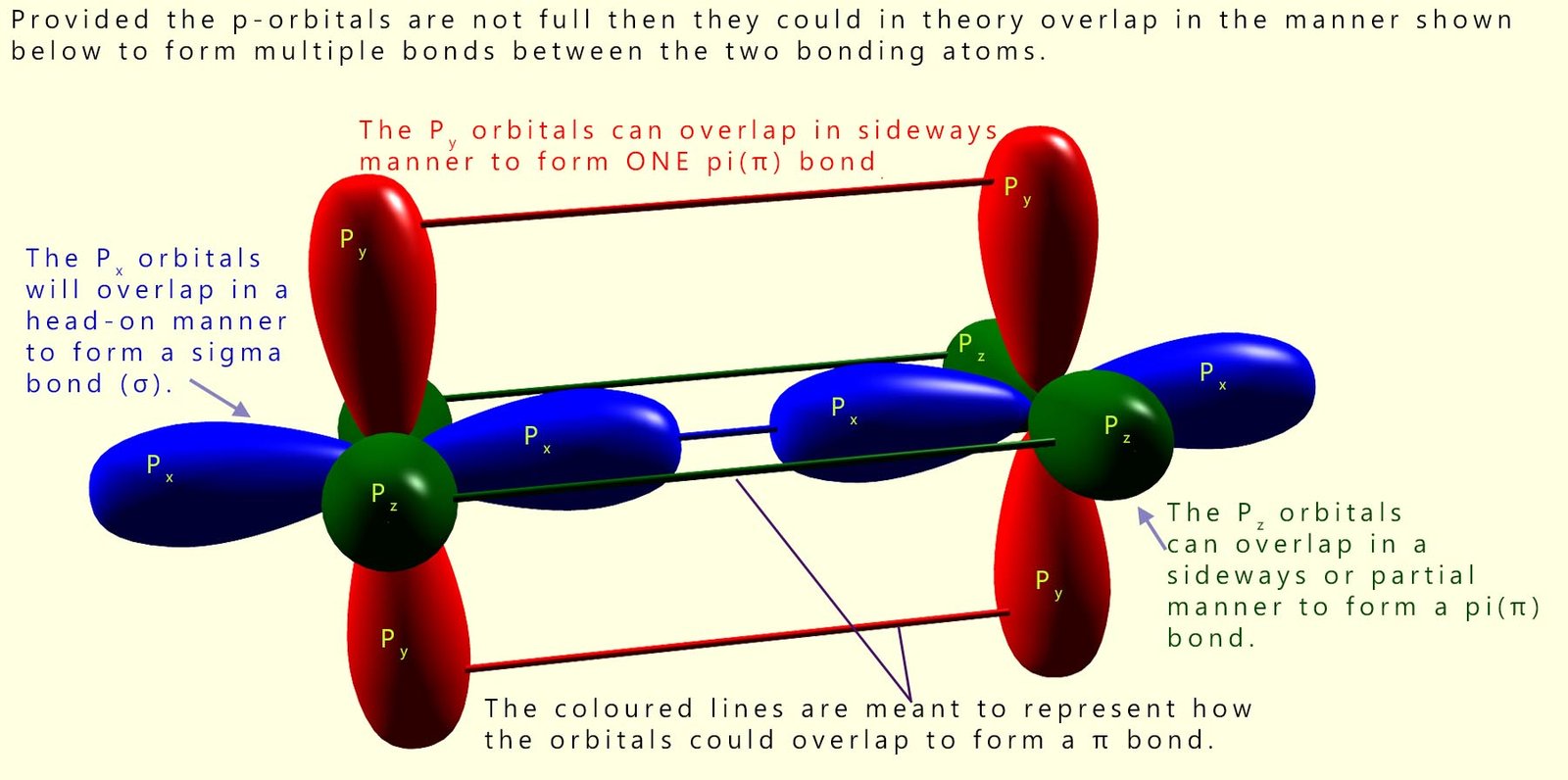
A possible way in which p-orbitals which contain only one electron could overlap and form sigma (σ) and pi(π) covalent bonds is shown below; in the diagram the Px orbitals will overlap in a head-on manner to form a sigma bond (σ) while the Py and Pz will partially overlap to form two pi(π) bonds.
Click the button below to try the quick quiz to review your understanding of sigma (σ) and pi (π) bonds.
Click whether each statement describes a sigma (σ) bond or a pi (π) bond.
Formed by head-on overlap along the internuclear axis
Restricts rotation about a carbon–carbon double bond
Present in every single, double and triple covalent bond
Formed by sideways overlap of p-orbitals
Generally the stronger type of covalent bond
| Bond type | How the bond forms | Orbitals involved | Strength 💪 | Key exam points 🧠 |
|---|---|---|---|---|
| Sigma (σ) | Head-on overlap along the internuclear axis | s–s, s–p or p–p | Stronger |
Present in all single, double and triple bonds Allows free rotation |
| Pi (π) | Sideways overlap above and below the axis | p–p only | Weaker |
Only found in multiple bonds Restricts rotation 🔄 |
| Bond type | Sigma (σ) | Pi (π) |
|---|---|---|
| Single bond | 1 | 0 |
| Double bond | 1 | 1 |
| Triple bond | 1 | 2 |
Click the button below to try the quick quiz to review your understanding of sigma (σ) and pi (π) bonds in molecules with multiple bonds.
For each molecule, pick the correct number of
sigma (σ) and
pi (π) bonds.
Tip: every double or triple bond contains exactly one σ.
| Exam pitfall ⚠️ | Why it loses marks ❌ | Correct exam thinking ✅ |
|---|---|---|
| Saying “orbitals bond together” | Orbitals do not bond – this wording shows poor understanding | Say overlap of orbitals forming a covalent bond |
| Forgetting the σ bond in multiple bonds | Students often count only π bonds in double or triple bonds | Every multiple bond contains exactly one σ bond |
| Saying π bonds are stronger than σ bonds | Shows confusion between individual bond strength and total bond strength | σ bonds are stronger due to greater orbital overlap |
| Mixing up head-on and sideways overlap | Leads to incorrect identification of σ and π bonds | σ = head-on, π = sideways |
| Incorrect explanation of restricted rotation | Students mention “double bonds are rigid” without explanation | Rotation would require breaking the π bond |