
Before reading this page, it helps to have a good understanding of electrophilic addition mechanisms. If you need a quick recap, click here.
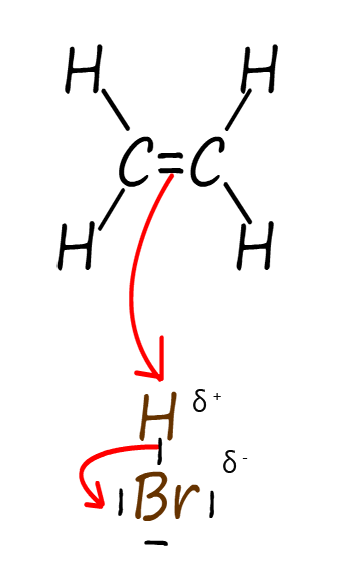
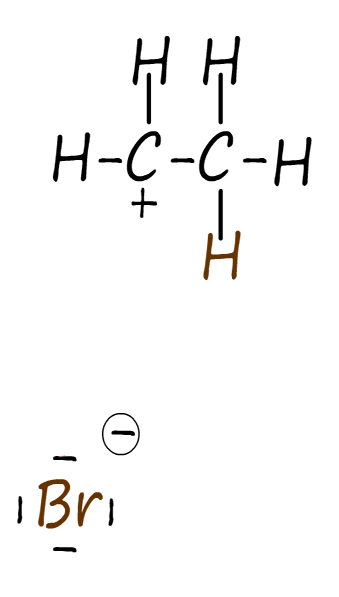
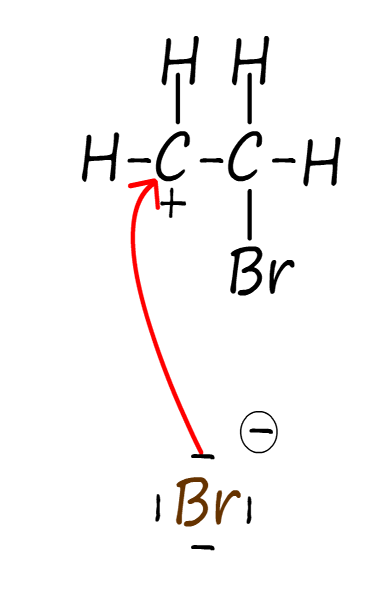
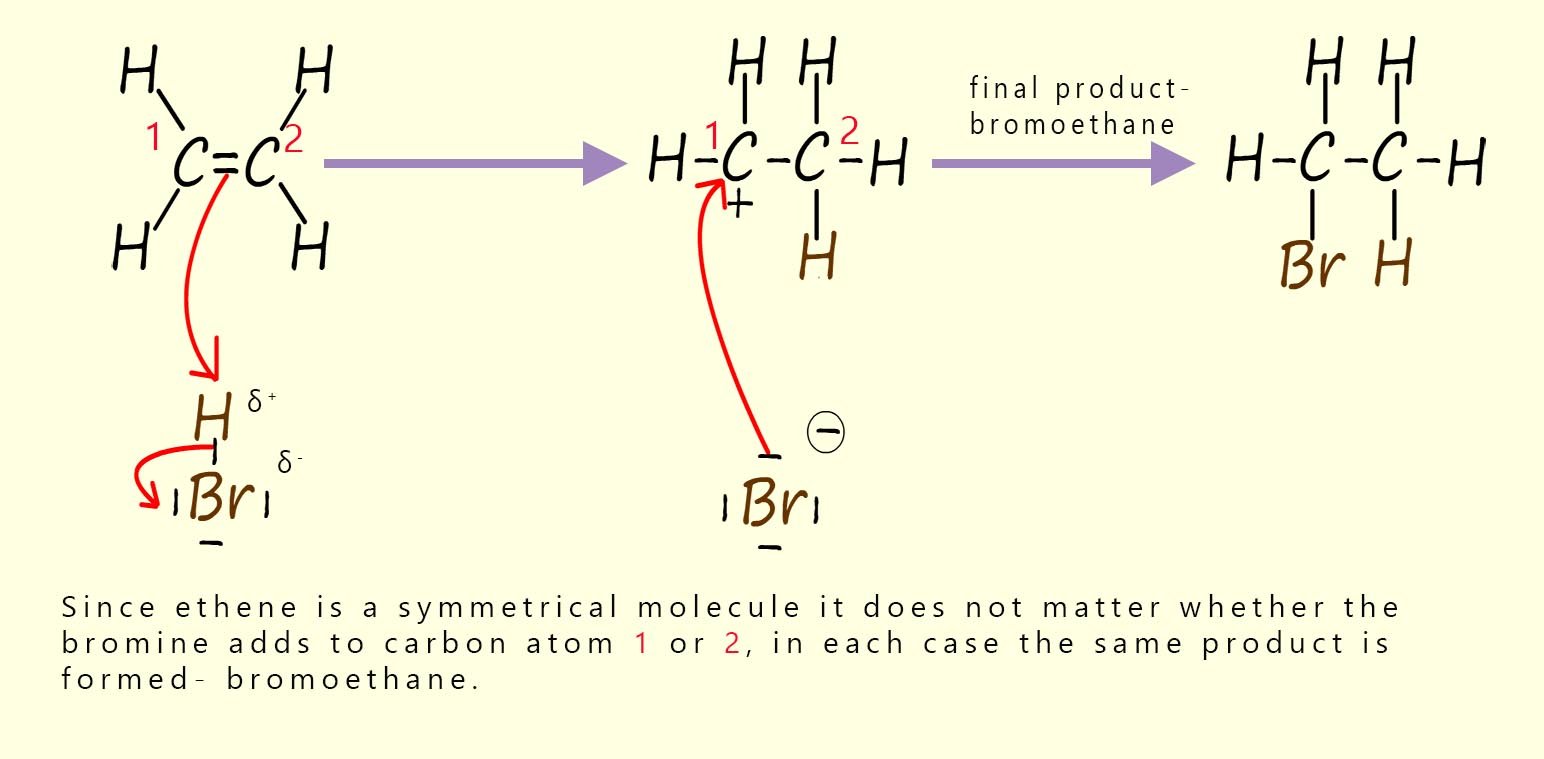
Electrophilic additions reactions always follow the same basic outline, for example consider the addition of hydrogen bromide gas (H-Br) to the alkene ethene to form the halogenalkane (haloalkane) molecule bromoethane. The mechanism for this electrophilic addition reaction can be considered as proceeding through the three basic steps; these steps are outlined in the table below:
| Step 1 | Step 2 | Step 3 |
|---|---|---|
|
|
|
| Step 1: The two electrons in the pi bond in the ethene carbon–carbon double bond (C=C) attack the electrophile. The electrophile in this case is the δ+ hydrogen atom in the H-Br molecule. | Step 2: Addition of a hydrogen atom to the alkene ethene results in the formation of a carbocation intermediate. Now when the H-Br bond breaks the bromine atom will gain both the electrons in this bond, this results in the formation of a bromide ion (Br-). | Step 3: The intermediate carbocation is then attacked by a nucleophile, forming the final product. The nucleophile in this case will be the bromide ion (Br-). This results in the formation of the final product; the halogenalkane (haloalkane) bromoethane. |
The three steps described in the table above are set out below to reveal the full mechanism for this electrophilic addition reaction. You should be able to clearly see how all three of the individual steps flow one after the other to form the final product. The mechanism begins with the electrophile; which is the partial positively charged hydrogen atom (δ+) in a molecule of hydrogen bromide being attacked by the pi bond electrons in the C=C bond of the alkene. This results in the formation of a carbocation; which is itself an electrophile; and a negatively charged bromide ion (Br-). In the final step the bromide ion (Br-) then uses one of its lone pairs of electrons to form a new covalent bond to the positively charged carbocation, so it is acting as a nucleophile. This is outlined below:
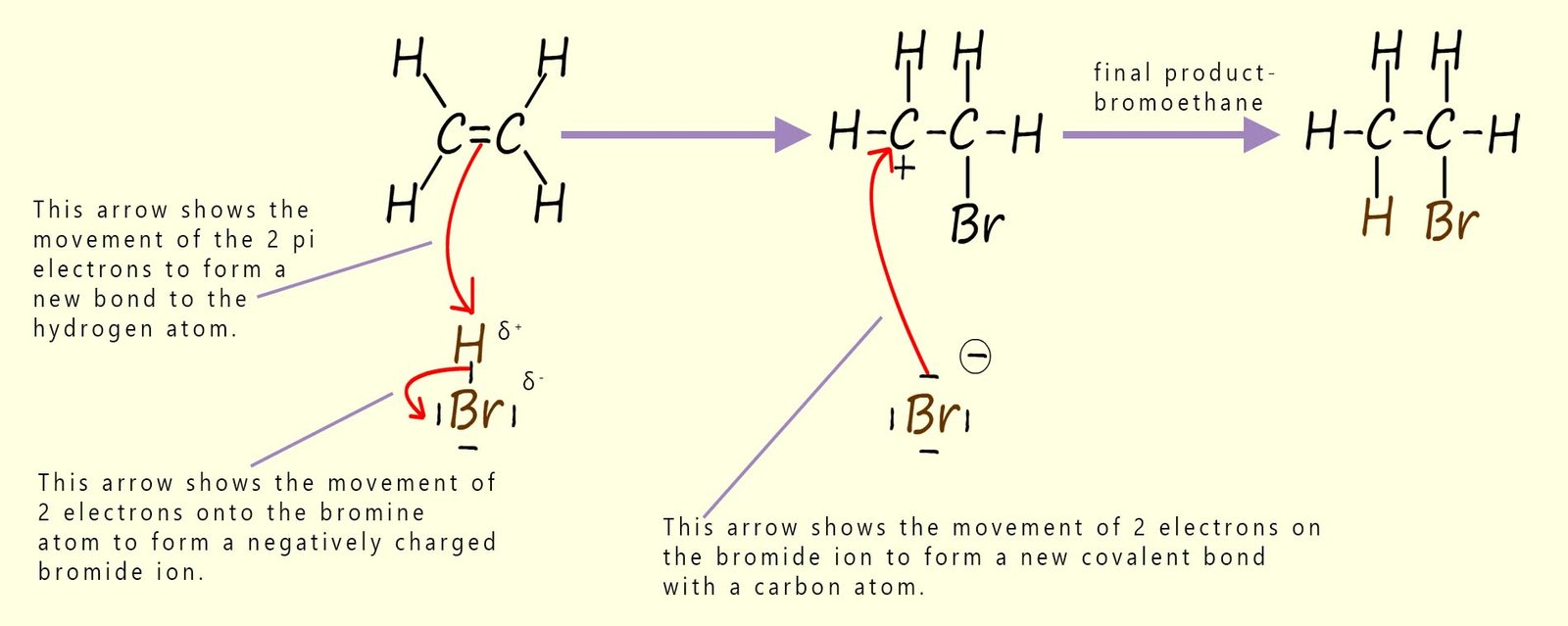
Test your understanding of electrophilic additions reactions, answer the question below:
In the electrophilic addition of hydrogen bromide to an alkene, which step happens first?
For the moment let’s focus on the intermediate carbocations formed in step 2 of the electrophilic addition mechanism shown above. Depending on the alkene you start with the carbocation formed during an electrophilic addition reaction can be a primary, secondary or tertiary one. The differences between these types of carbocations are discussed in the table below. At first glance the differences between these carbocations may appear quite small but they have a big effect on how an electrophilic addition reaction proceeds and on the final product formed. The table shows the structures of typical primary, secondary and tertiary carbocations.
| Primary carbocation | Secondary carbocation | Tertiary carbocation |
|---|---|---|
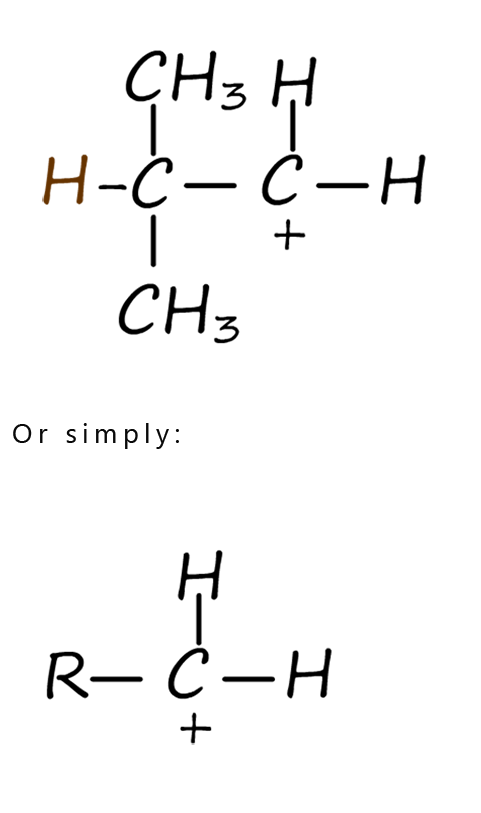
|
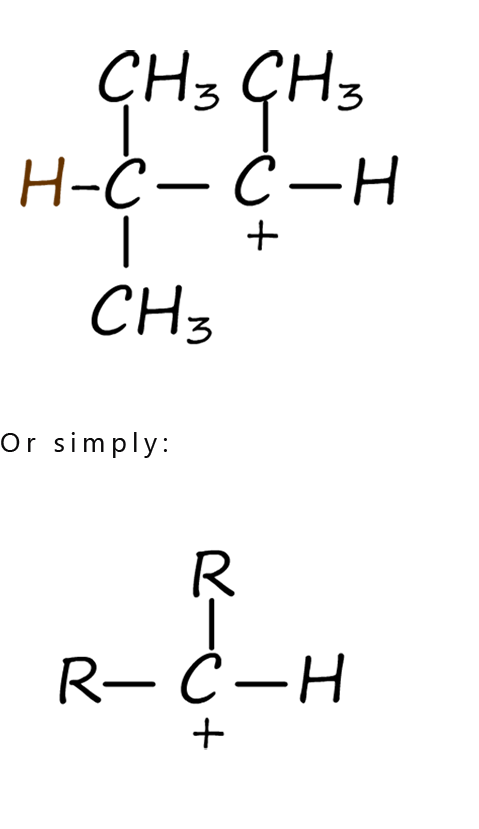
|
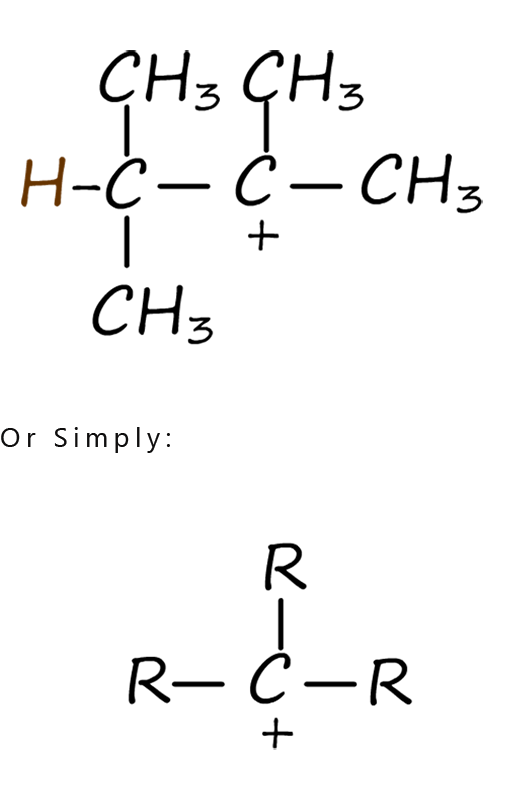
|
| In a primary carbocation the positively charged carbon atom is attached to two hydrogen atoms and one alkyl group (-R). | In a secondary carbocation the positively charged carbon atom is attached to one hydrogen atoms and two alkyl groups (-R). | In a tertiary carbocation the positively charged carbon atom is attached to three alkyl groups(-R). |
So why are we focusing in on these intermediate carbocations and why are they important? Well in the first example above, a molecule of hydrogen bromide adds to the unsaturated alkene ethene. Ethene is a symmetrical molecule, so it makes no difference to the final product which carbon atom ends up forming the intermediate carbocation.
Answer the questions in the quick quiz below to test your knowledge on the stability of carbocations:
In an electrophilic addition reaction, the carbocation that forms most easily
is usually the one with the lowest activation energy.
Choose the best answer for each question.
Which carbocation is usually most stable?
Which carbocation usually needs the highest activation energy to form?
Which statement is correct?
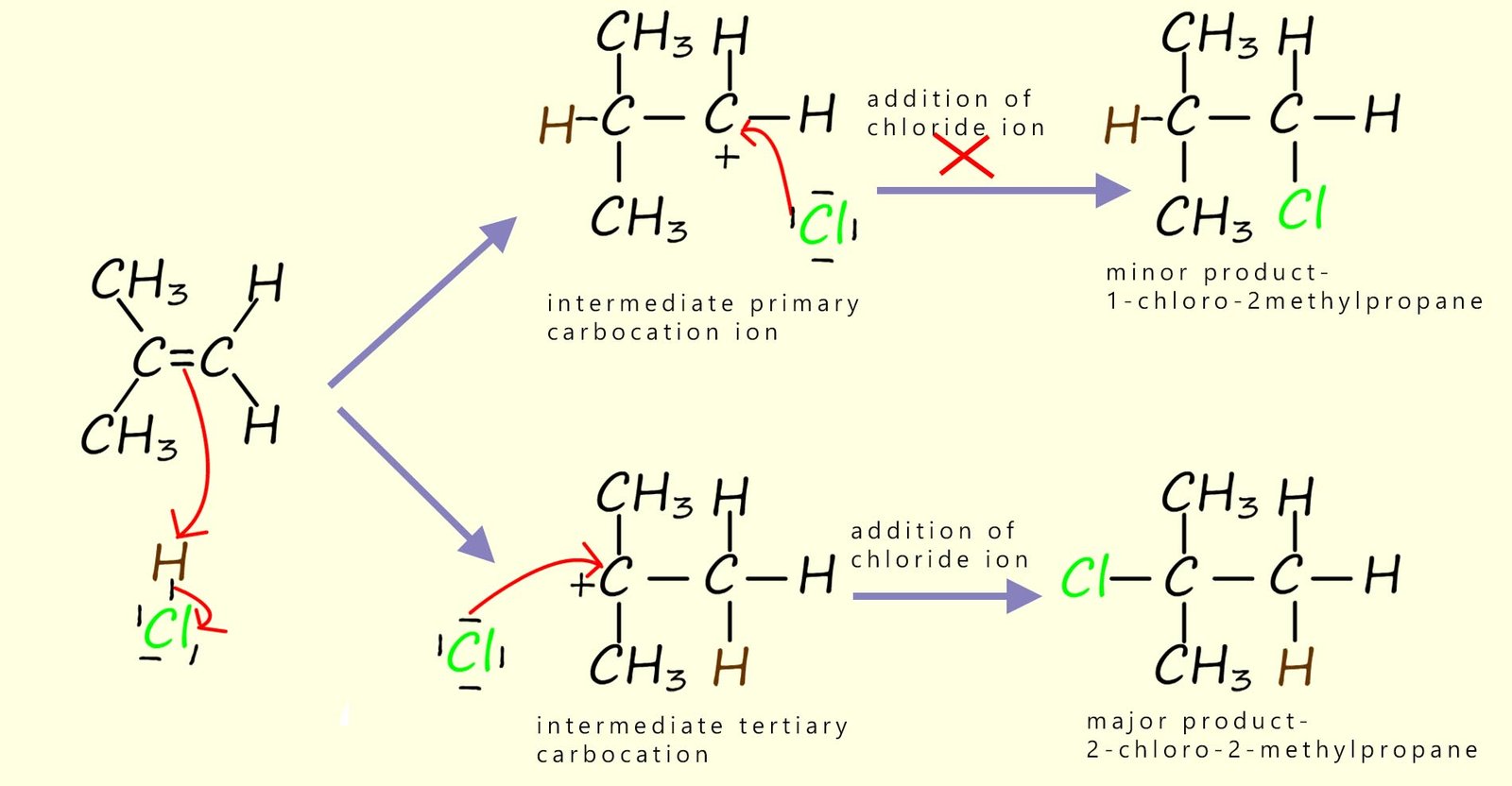
However if we start with an unsymmetrical alkene such as 2-methylpropene as shown below, then it does make a difference which carbon atom in the C=C bond ends up forming the intermediate carbocation. In the diagram below a molecule of hydrogen chloride gas adds to our unsymmetrical alkene molecule. Now you can see that depending on which carbon atom the hydrogen from the H-Cl adds to can result in the formation of two possible carbocation intermediates; one possible intermediate is a primary carbocation while the other carbocation that can form is a tertiary carbocation. Each of these different intermediate carbocations then goes on to form two different products.
Primary carbocations are much less stable than secondary carbocations, which in turn are less stable than tertiary carbocations ⚖️
One explanation is the inductive effect of alkyl groups. Alkyl groups release (donate) electron density towards a carbocation, helping to stabilise the positive charge 💡
The more alkyl groups attached that are attached to the carbocation the stronger this effect tends to be and the more stable the carbocation becomes ✅
However of the two possible products of the above reaction the 1-chloro-2-methylpropane is not produced in any significant amounts. Why is this? The simplest explanation is that the intermediate carbocations shown above are not equally stable and so some require more energy to form than others. In other words, they have a higher activation energy. In fact we can summarise the relative stabilities of primary, secondary and tertiary carbocations as:
So, in our example above the major product of the reaction will be 2-chloro-2-methylpropane since this compound forms via a route that has a relatively stable tertiary carbocation as an intermediate, whereas the alternative product ;1-chloro-2-methylpropane; would require formation of an unstable primary carbocation.
By studying many electrophilic addition reactions, the Russian chemist Vladimir Markovnikov suggested that:
Markovnikov suggested that in the addition of hydrogen halides to unsaturated alkene molecules, the electrophilic hydrogen becomes attached to the carbon atom with the fewer alkyl substituents (equivalently, the carbon atom that already has more hydrogen atoms).
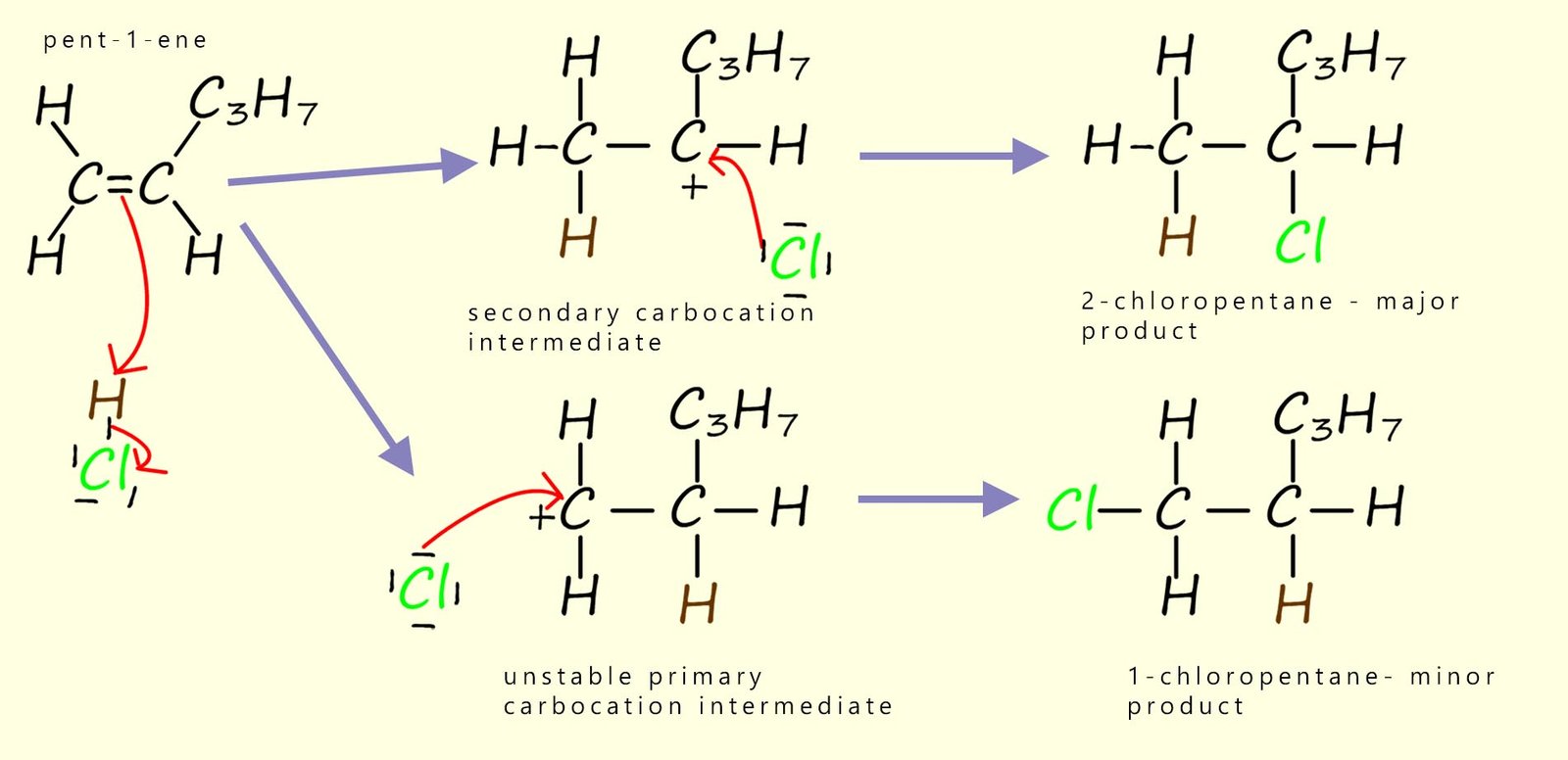
This rule can also be explained by saying that, in the addition of HX to an alkene, the more substituted carbocation intermediate is more likely to form because it is more stable ⚖️
In the example above using HCl and 2-methylpropene, this reaction can proceed via either a tertiary or a primary
carbocation. The tertiary carbocation is so much more stable than the primary carbocation
so one product is formed predominantly- that is the product that goes through the tertiary carbocation.
Try the quick quiz below to test your understanding of Markovnikov's rule:
Use Markovnikov's rule and your understanding of carbocation stability to choose the major product.
When HCl adds to pent-1-ene, what is the major product?
When HBr adds to 2-methylpropene, what is the major product?
Which statement best explains why the major product forms?
Markovnikov questions usually look simple… and that’s why they’re dangerous 😅 Most mistakes come from mixing up which carbon gets the hydrogen, or ignoring carbocation stability.
Quick self-check (typical exam traps) ✅
| Key idea | What happens | Why it matters |
|---|---|---|
| ⚡ Electrophilic addition | The pi bond in an alkene attacks an electrophile, forming a carbocation intermediate. | This explains why alkenes react readily with electrophiles. |
| 🧩 Carbocation intermediates | The intermediate carbocation formed can be primary, secondary or tertiary. | Different carbocations lead to different possible products. |
| ⚖️ Stability and activation energy | Tertiary carbocations are most stable, followed by secondary then primary. | More stable carbocations require a lower activation energy to form and are more likely intermediates. |
| 🧠 Markovnikov’s rule | In the addition of HX to an unsymmetrical alkene, hydrogen attaches to the carbon with more hydrogen atoms. | This rule predicts the major product of the reaction. |
| 🏁 Major vs minor products | The major product forms via the more stable carbocation. | This links the mechanism directly to the observed product ratio. |
