

Stereoisomers have the same molecular formula and structural formula but the atoms are arranged differently is space. There are two types of stereoisomers:

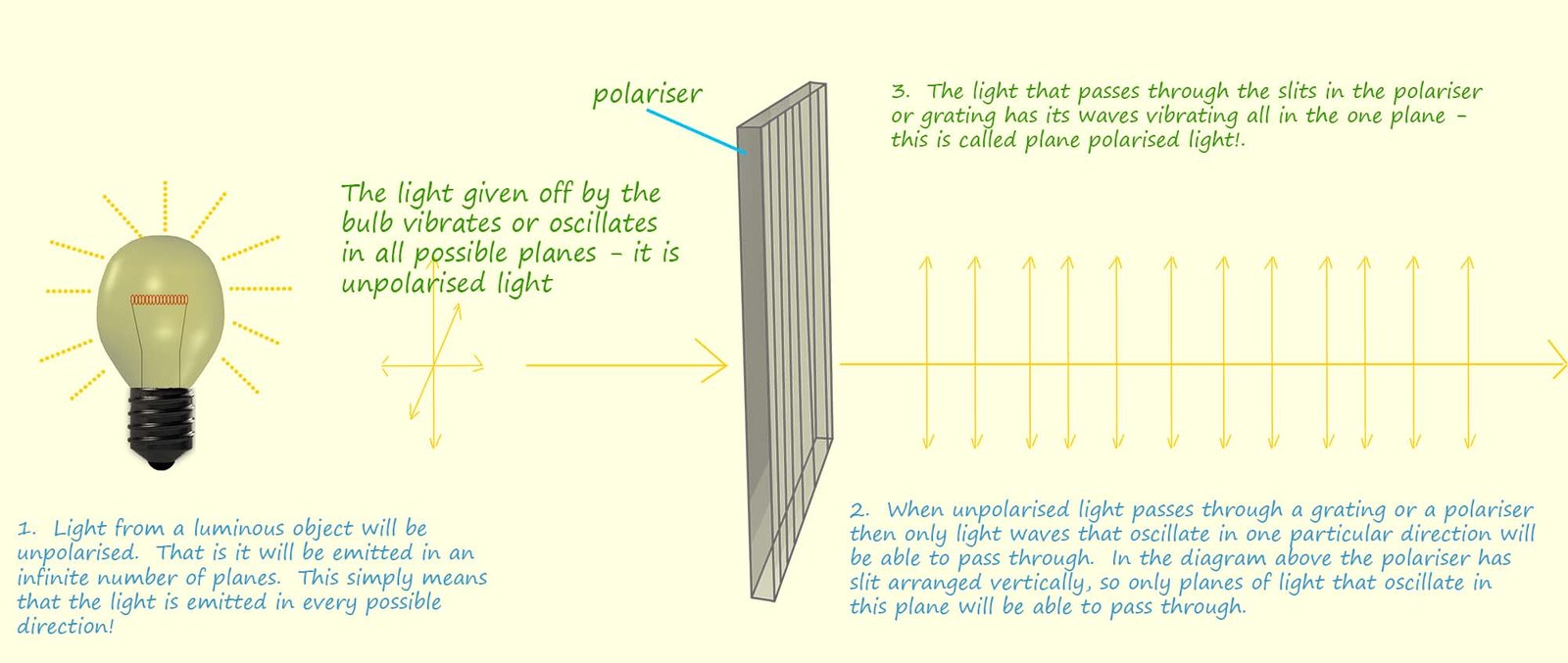
The light that we see everyday objects by is unpolarised, this simply means that the waves of electromagnetic
radiation (visible light) that for example a light bulb emits travel out from the bulb in
every direction or plane; this is shown
in the diagram below. However when this unpolarised light passes through a
polariser only light waves that are
vibrating in one particular plane can pass through, all the other
planes of light are blocked. Think of the
polariser much
like a simple drain cover with slits all running parallel; if you drop an object down through this drain cover
then only objects that fall parallel to the slits in the drain cover will be able to get through!
The light that passes through the polaroid material (the same material used in sunglasses) is
said to be plane
polarised light, that is all the light waves are vibrating in only one
single plane.
The image below outlines how a polariser works and how the light that passes through the polariser contains waves that are all oscillating in a single plane. This process is called linear polarisation, and the resulting light is referred to as polarised light.
If a beam of plane polarised light is passed through certain organic molecules something remarkable happens, the beam
of plane polarised light is
rotated, either to the left or to the right. Molecules
which rotate
plane polarised light are said to be
optically active molecules.
In the diagram below you should be able to see the
unpolarised light waves travelling from the bulb, these then enter the
polariser and only light waves vibrating
parallel to the slits in the
polariser pass through it. Next the plane polarised light
enters a sample tube
containing an optically active compound. In the diagram the
optically active compound rotates
the plane
polarised light by a few degrees. The light waves now meet another
polariser or analyser. The analyser is simply
a piece of polaroid film, much like the polariser. However when the rotated light waves meet it they
will not be able to
pass through until the analyser is rotated
by the same angle that the plane polarised light
was rotated by the
optically active sample in the tube. By simply measuring how
much the analyser was rotated we can measure how much the
plane polarised light was rotated by the
optically active molecules in the sample tube.

As well as measuring how much plane polarised light is rotated after passing through the sample tube we can also determine whether the plane polarised light is rotated clockwise or anti-clockwise. Some optically active molecules rotate plane polarised light anti-clockwise, these molecules are said to be levorotatory. Optically active molecules which rotate plane polarised light clockwise are said to be dextrorotatory. By convention anti-clockwise rotation is also give the minus sign (-) while clockwise rotation is given the positive sign (+), e.g. glucose is an optically active molecule, (+)-glucose is dextrorotatory and will rotate plane polarised light clockwise, while (-)- glucose is laevorotatory and will rotate plane polarised light anti-clockwise.
How do we know which molecules will rotate plane polarised light? Well in A-level chemistry we can say that all organic molecules with an asymmetric carbon atom will rotate plane polarised light. What this means in simple terms is that an organic molecule with a carbon atom bonded to four different groups will be optically active. This idea is highlighted in the image below:

Can you think of any ways in which your right and left hand are similar and also different from each other? There is
the obvious fact for example that each of your hands have 5 fingers but if you are in a rush and you put a left handed
glove on your right hand and the right handed glove on your left hand you would immediately know that something was not
quite right! It's a similar story with your feet, if you put the wrong shoes on the wrong feet then by the end of the day
you will have sore feet! So what is the relationship between your feet and your hands? Well they are
mirror images of each other.
It is not possible to place your right hand on top of your left hand and get all your fingers and thumbs to match up. Well
its the same with optically active molecules.
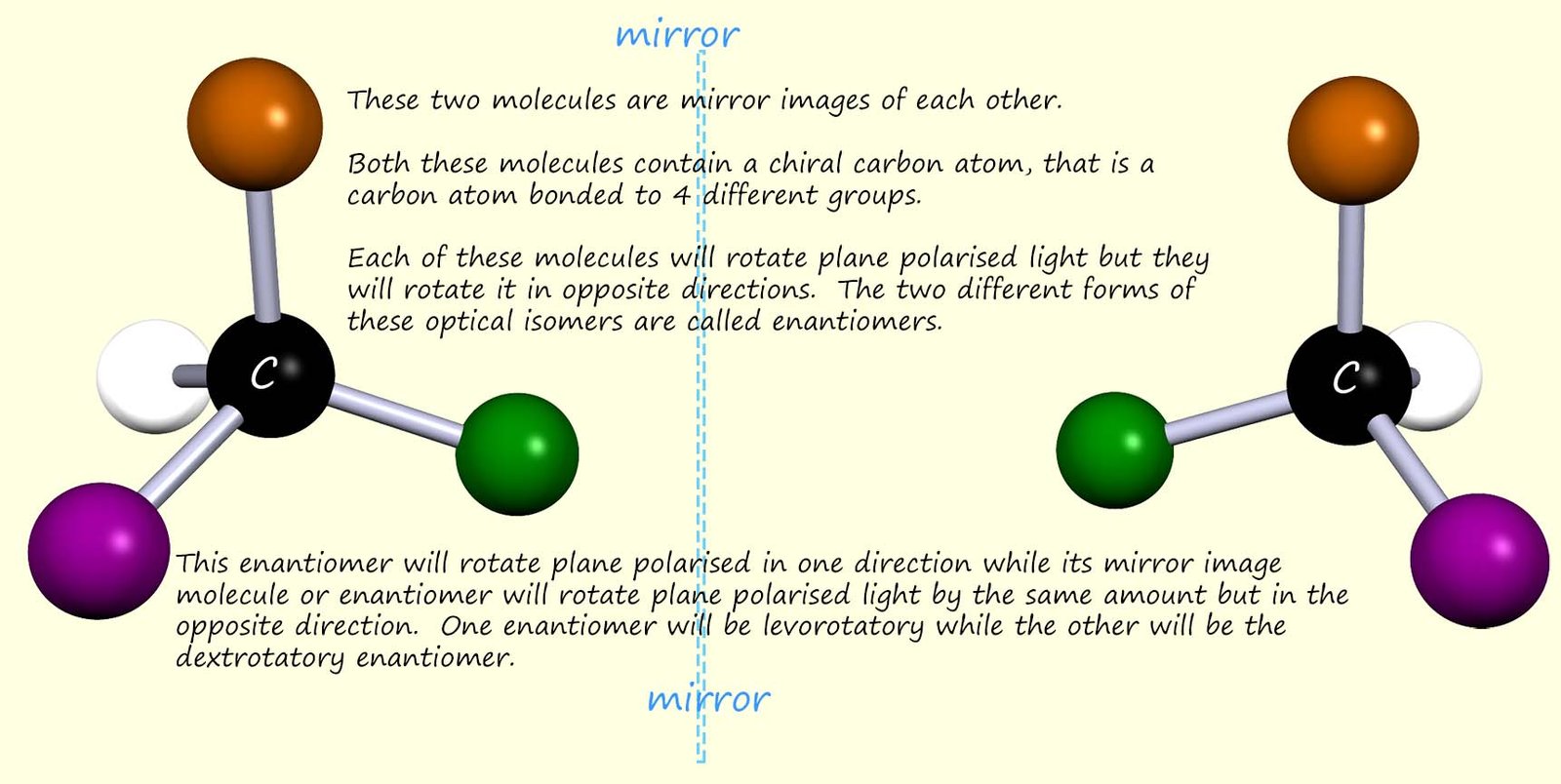
Mirror images forms of molecules which contain
four different groups attached to
a carbon atom, that is the carbon atom will be asymmetrical, will be
non-superimposable onto each other. These two mirror image forms of a
chiral molecule are called
enantiomers.
If a molecule is unsymmetrical
and contains no planes of symmetry then it will be non-superimposable on its mirror
image, these molecules are chiral
and will be optically active.
The two mirror image forms of an optically active molecule are
called enantiomers. Each of these mirror
image molecules or enantiomers will be
optically active and will rotate
plane polarised light, but
each enantiomer will rotate
plane polarised light in equal but opposite directions. This is shown in the diagram below:
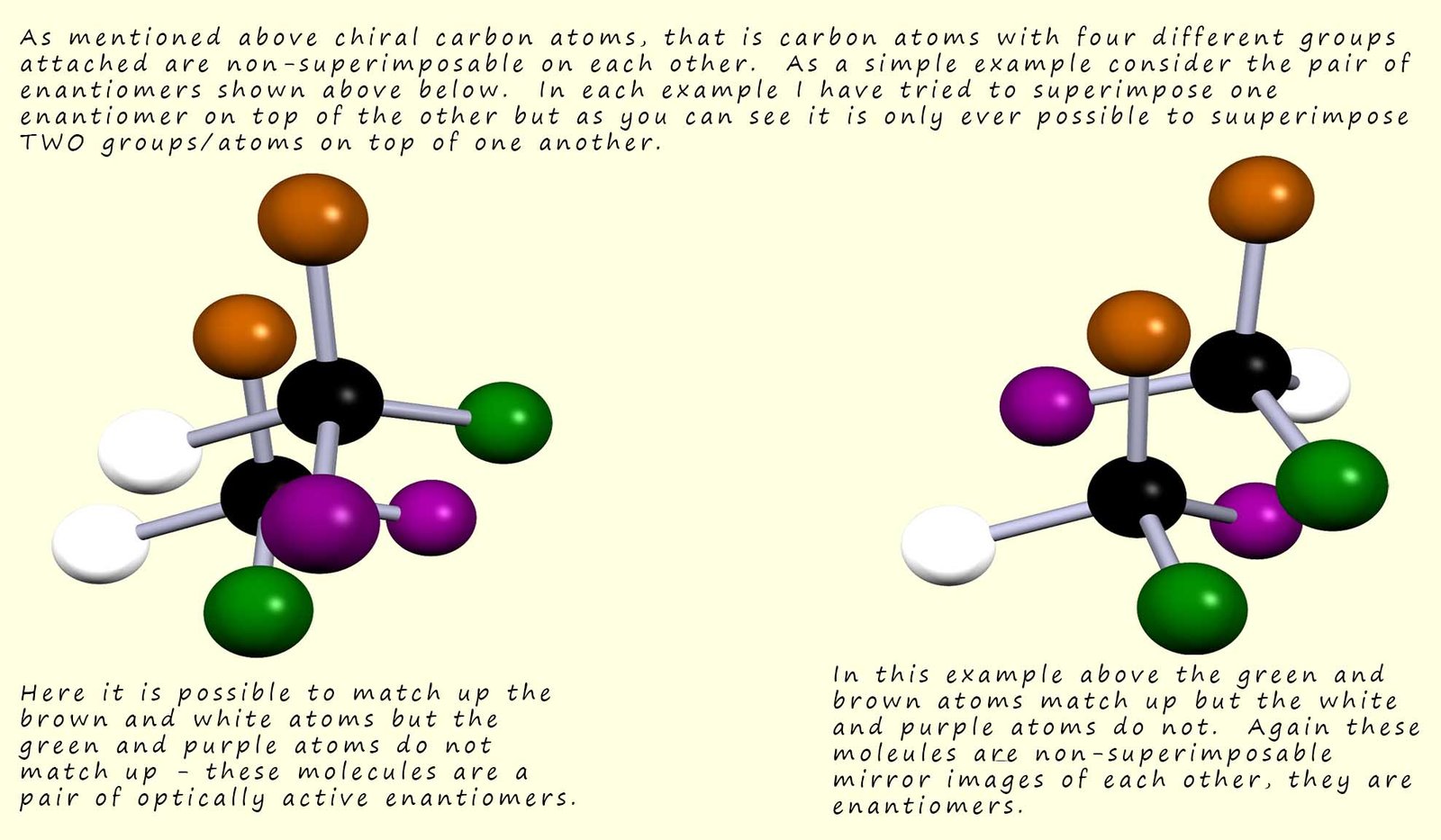
No matter how hard you try you cannot superimpose one enantiomer
on top of another, in the examples below it is only ever
possible to match-up TWO of the four groups on the central chiral carbon atom.

Many naturally occurring molecules exist as single
enantiomers. This is mainly due to the fact that many natural
processes in living organism involve enzymes and since
enzymes are stereospecific they can readily distinguish one
enantiomer from another. It is highly likely for example that
one enantiomer will fit nicely into an
active site in
an enzyme but the corresponding mirror image molecule, the
other enantiomer is unlikely to fit into the
enzyme's active site.
Almost all the chemical reactions that you are likely to carry out are not stereospecific and it is almost certain that
any synthesis will produce equal amounts of the two enantiomers, if the molecules you aim to produce are indeed are chiral. If a mixture of the two enantiomers are present in
equal
amounts then this mixture will be optically inactive and will not
rotate plane polarised light.
This is simply because if equal amounts of the
laevorotatory and dextrorotatory enantiomers are present then
the effects of the equal and opposite amount of rotation of
any plane polarised light will simply cancel each other out. Solutions
which contain
equal amounts of both enantiomers
are called racemic mixtures or a
racemate and they are optically inactive.
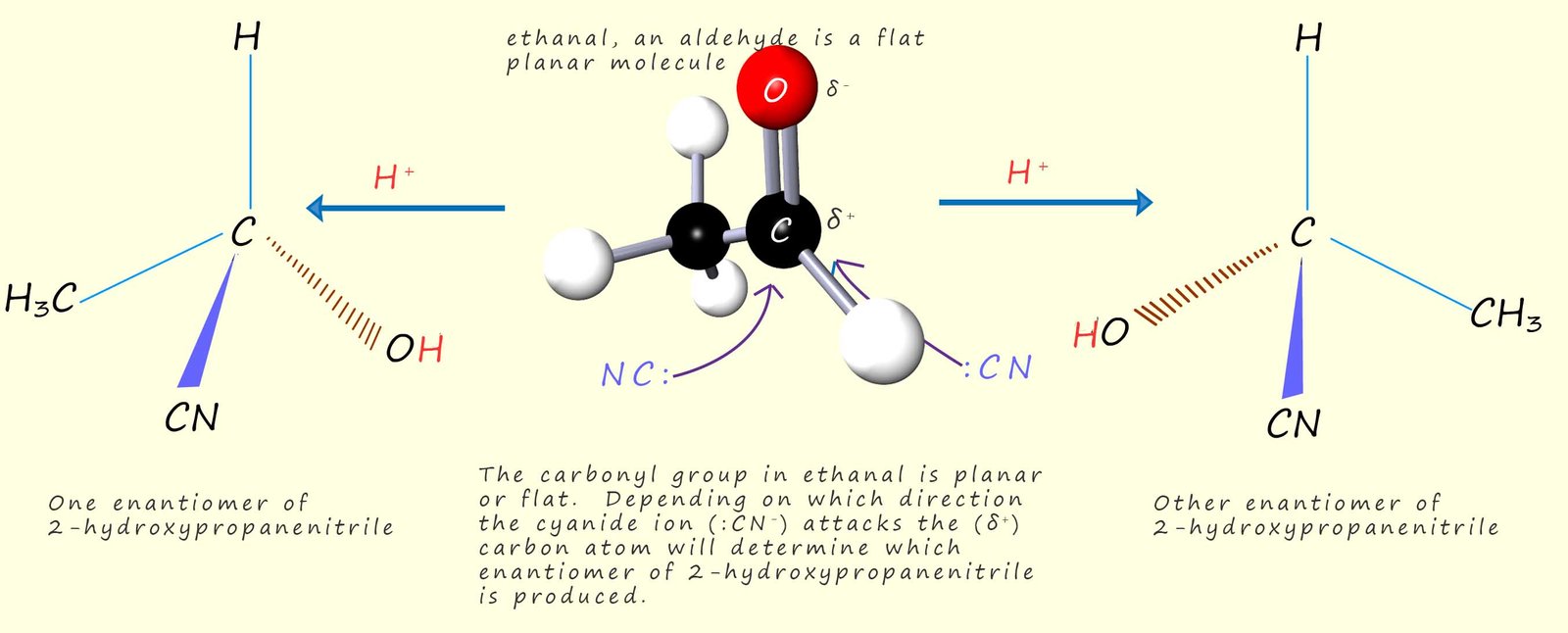
As an example consider the synthesis of the optically active compound 2-hydroxypropanoic acid. This compound can be prepared by the nucleophilic attack of a cyanide ion (:CN-) on a molecule of ethanal. Ethanal is a flat planar molecule and the cyanide ion (:CN-) can attack the δ+ carbon atom in the carbonyl group in a molecule of ethanal. Since the nucleophilic cyanide ion can attack the carbonyl group with equal probability from either side of the carbonyl group an equal mixture of the two possible enantiomers is produced, that is a racemic mixture of the (-) and (+) enantiomers of 2-hydroxypropanoic acid are formed. This is outlined in the diagram below:

In the lab it is unlikely that you will get the opportunity to use just one particular enantiomer of an optical active compound. The reasons for this are fairly simple:
Drugs work by changing or interfering with a chemical reaction inside the body. Some drugs do this by binding to an active site on a particular enzyme or receptor molecule. This means that the drug must be the exact shape to fit into the active site in the enzyme. If one enantiomer fits into the active site it is highly unlikely that the mirror image molecule or other enantiomer will fit. Indeed it maybe that this unwanted enantiomer could bind to another active site elsewhere which could result in harmful side-effects, these side-effects could be minor or may even result in the death of a patient. Two examples of this are:
Thalidomide was a drug
developed by the German pharmaceutical company Chemie Grünenthal GmbH.
Thalidomide was prescribed by a doctor to treat conditions such as colds and flu and for relieving
the symptom of nausea and morning sickness often experienced in the early stages of pregnancy.
Thalidomide
is an optically active substance with chiral centres present in the molecule.
While one of the enantiomers of this drug had the desired
therapeutic effects the other enantiomer caused
serious birth defects in babies. The drug
only affected
the unborn embryo if the mother took thalidomide between 20 and 37 days after conception.
Thalidomide
resulted in the death of over 2000 unborn babies and
caused approximately 10 000 babies to be born with serious limb
defects and defective organs.
Seldane is another drug where one
enantiomer is an effective antihistamine used to treat the symptoms of
hay fever and congestion while the mirror image enantiomer can cause serious and fatal heart defect in some
patients.
Considering the two drug examples mentioned above most new drugs prescribed now are likely to consist of one enantiomer. This has a number of benefits:

Not all drugs consist of single enantiomers. Ibuprofen is an analgesic which is taken to relieve the symptoms of muscle pain, migraines, period pains and to treat the symptoms of colds and flu. The ibuprofen molecule has a single chiral carbon atom so it consists of a pair of optical isomers. The (+)-enantiomer acts much more quickly to relieve pain than its mirror image enantiomer. Luckily once inside the body the less active (-)-enantiomer is converted into the more active (+)-enantiomer, this means that the whole dose taken is active and it is not necessary to take a double dose to achieve the desired therapeutic effect.