
Molecules with four different atoms or groups attached to a carbon atom are optically active, they will
rotate plane polarised light. Carbon atoms with four different atoms or groups attached
are also said to be chiral or asymmetric.
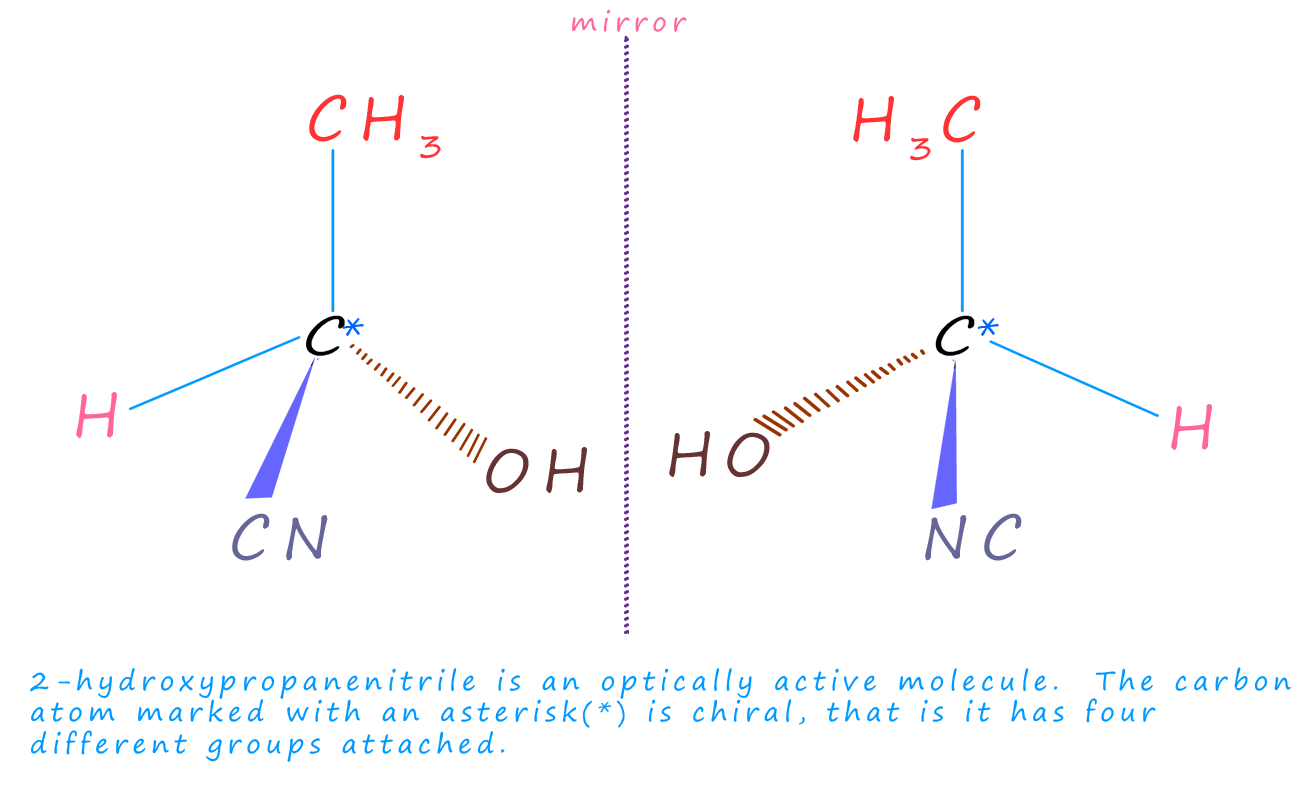
The molecule 2-hydroxypropanenitrile (lactic acid) is shown in the image below.
2-hydroxypropanenitrile contains one chiral carbon atom which is highlighted by an asterisk (*). This carbon atom
has four different groups attached, meaning it will exist then as a pair of optically active
enantiomers.
Enantiomers are mirror image forms of
optically active molecules; they are non-superimposable mirror images of
each other.
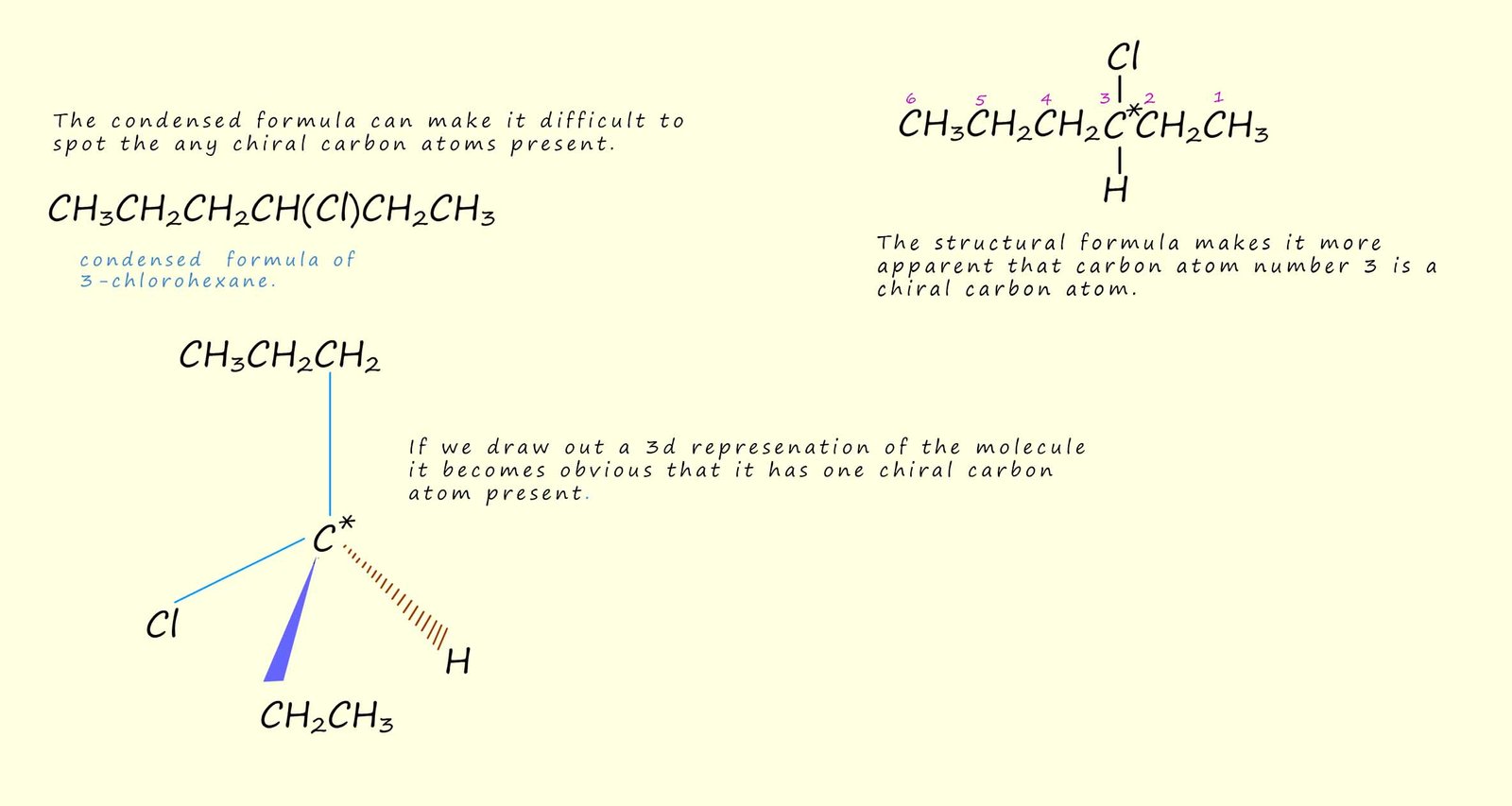
In small simple molecules such as lactic acid shown above it can be fairly easy and quick to find any chiral carbon atoms present, however with larger molecules and especially in molecules which consist of rings it is not always immediately obvious if a chiral carbon atom is present or not. Consider the example shown below, here the optically active molecule 3-chlorohexane is chosen as a simple example. Now depending on which type of formula is shown for a particular molecule can determine how easy it is to determine if the molecule is optically active or not, for example the condensed formula can make it difficult to determine if there are any chiral carbon atoms present, while the structural formula can make it easier to identify the presence of chiral carbon atoms. However if the 3d structure of the molecule is drawn out it can make it relatively straight forward to identify any chiral carbon atoms present, as shown in the image below:
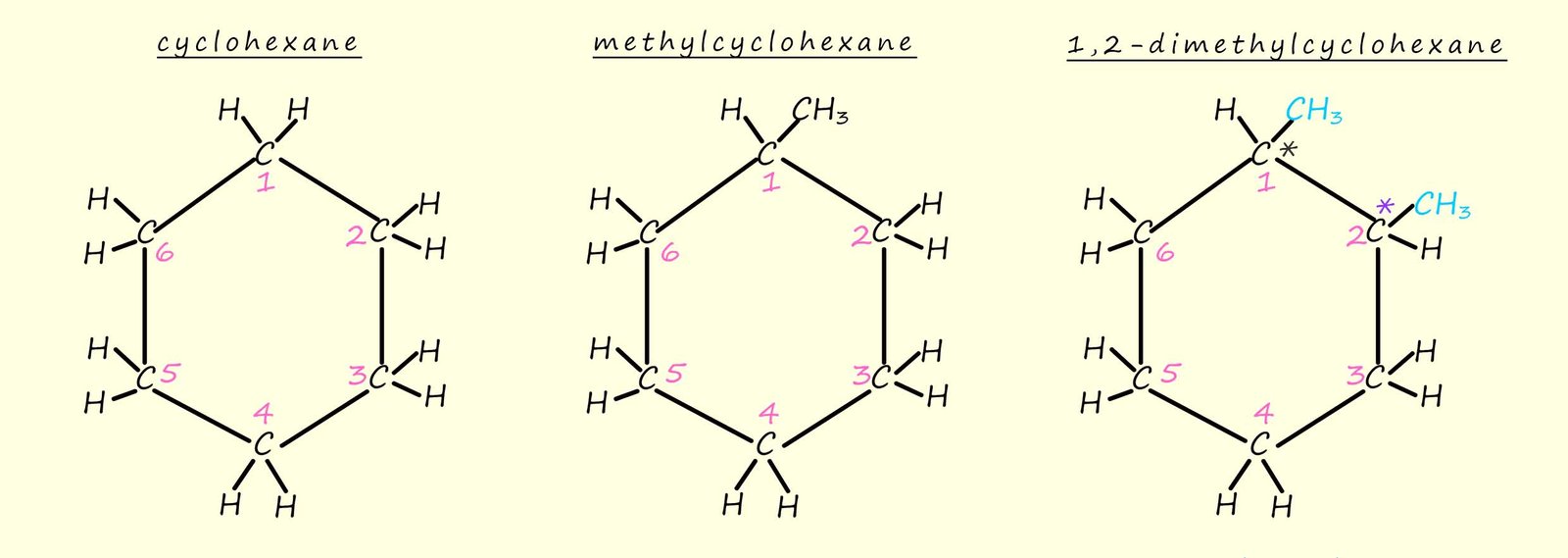
Finding chiral carbon atoms in ring or cyclic structures needs a bit more care. The trick to finding chiral centres in rings and cyclic molecules is to imagine parts of the ring structure as individual substituents. I have given one example below, out of the three molecules shown below only the last molecule, 1,2-dimethylhexane is the only one which contains a chiral carbon atom.