

 There are three basic methods which can be used to prepare aliphatic amines; these are:
There are three basic methods which can be used to prepare aliphatic amines; these are:
Aliphatic amines can be prepared by warming a halogenalkane with an excess of ammonia in a sealed vessel for a few minutes, using aqueous ethanol as a solvent. The reaction vessel needs to be sealed to prevent the gaseous ammonia escaping. Ammonia is not only a good base, due to the presence of the lone pair of electrons on the molecule it is also a good nucleophile. The mechanism for the nucleophilic substitution reaction between the halogenalkane bromoethane and ammonia is outlined below, here ammonia reacts with the halogenalkane bromoethane to form the primary amine ethylamine and the amine salt ammonium bromide is also formed.
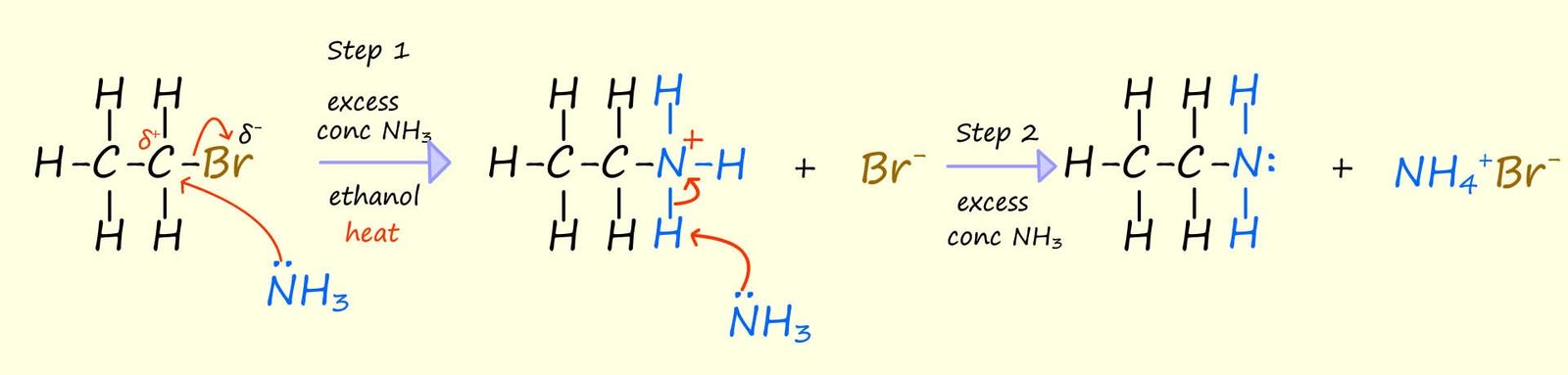
This nucleophilic substitution reaction proceeds via two steps:
We can easily write word and symbolic equations to show this reactions:
However this is not the end of the story for this reaction. If you follow the mechanism you can clearly see that
the ammonia molecule simply swaps a hydrogen atom for an alkyl group in this case an ethyl group (-CH3CH2) or
if a different halogenalkane was used; for example bromomethane
then a methyl group would replace a hydrogen on the ammonia
to form methylamine.
The problem with this method of preparing amines is the product of the reaction; the primary amine
ethylamine is a stronger base and a better nucleophile
than
ammonia; which was the initial starting material. So as the reaction proceeds the concentration of the ethylamine produced will
increase and it will take over from the ammonia as the nucleophile in step 1 of the reaction and as the base in step 2 to produce the secondary amine diethylamine; as outlined in the image below
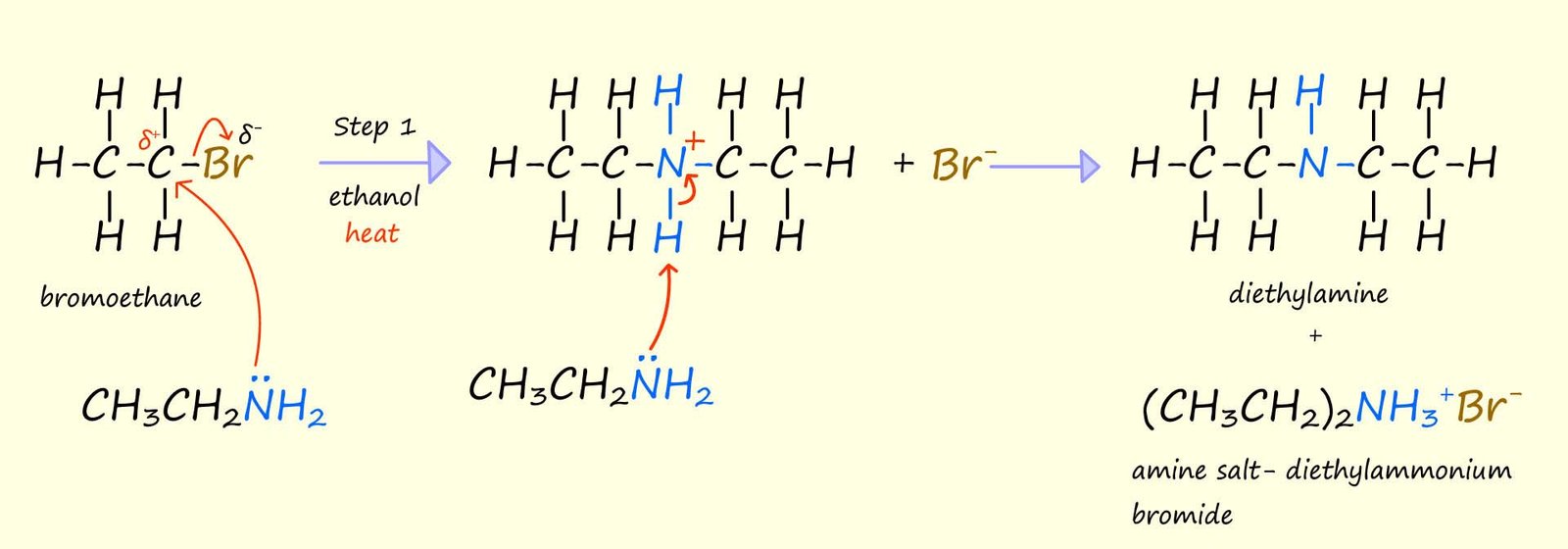
 I am sure you can see where this going! The product of the reaction above, the secondary amine
diethylamine is a better nucleophile
than the primary amine ethylamine. This means that as the
concentration of the secondary amine diethylamine increases
it will take over from the ethylamine and form the tertiary amine triethylamine.
Even here the reaction will not stop! The
triethylamine will continue to react with the bromoethane and form the quaternary ammonium salt where all
the hydrogen
atoms from the original ammonia molecule
have been replaced by -ethyl groups.
I am sure you can see where this going! The product of the reaction above, the secondary amine
diethylamine is a better nucleophile
than the primary amine ethylamine. This means that as the
concentration of the secondary amine diethylamine increases
it will take over from the ethylamine and form the tertiary amine triethylamine.
Even here the reaction will not stop! The
triethylamine will continue to react with the bromoethane and form the quaternary ammonium salt where all
the hydrogen
atoms from the original ammonia molecule
have been replaced by -ethyl groups.
This reaction leads to a mixture of products which ultimately reduces the usefulness of this reaction. We can
of course try to stop the
reaction at the first step and only produce the primary amine ethylamine, to stop the reaction here we
simply try to block the product of the reaction, the ethylamine from acting as a nucleophile and undergoing further reactions by using a large
excess of ammonia. This it is hoped by sheer weight of
numbers the ammonia molecules will
simply block the ethylamine and limit the
reaction to produce only the primary amine, ethylamine. However this will have some effect but a mixture of primary, secondary and tertiary amines and an amine salt will still be formed, albeit in reduced amounts.
However if the desired product of the reaction is the ammonium salt then the reaction mixture needs to be adjusted and a large excess of the halogenalkane should be used, this will ensure that there are plenty alkyl groups to substitute for the hydrogen atoms on the starting ammonia molecules.
A more efficient method of preparing amines which removes the problem of producing mixtures of amines is to firstly convert a halogenalkane into a nitrile and then reduce the nitrile to form an amine; that is:
Recall that nitriles contain the functional group R-CN. Nitriles are particularly useful in organic synthesis are they are one of the few ways in which it is possible to extend the carbon chain by 1 carbon atom. Nitriles are also reactive and are easily converted into other useful and reactive molecules including amines. The first two members of the nitriles homologous series are shown below:


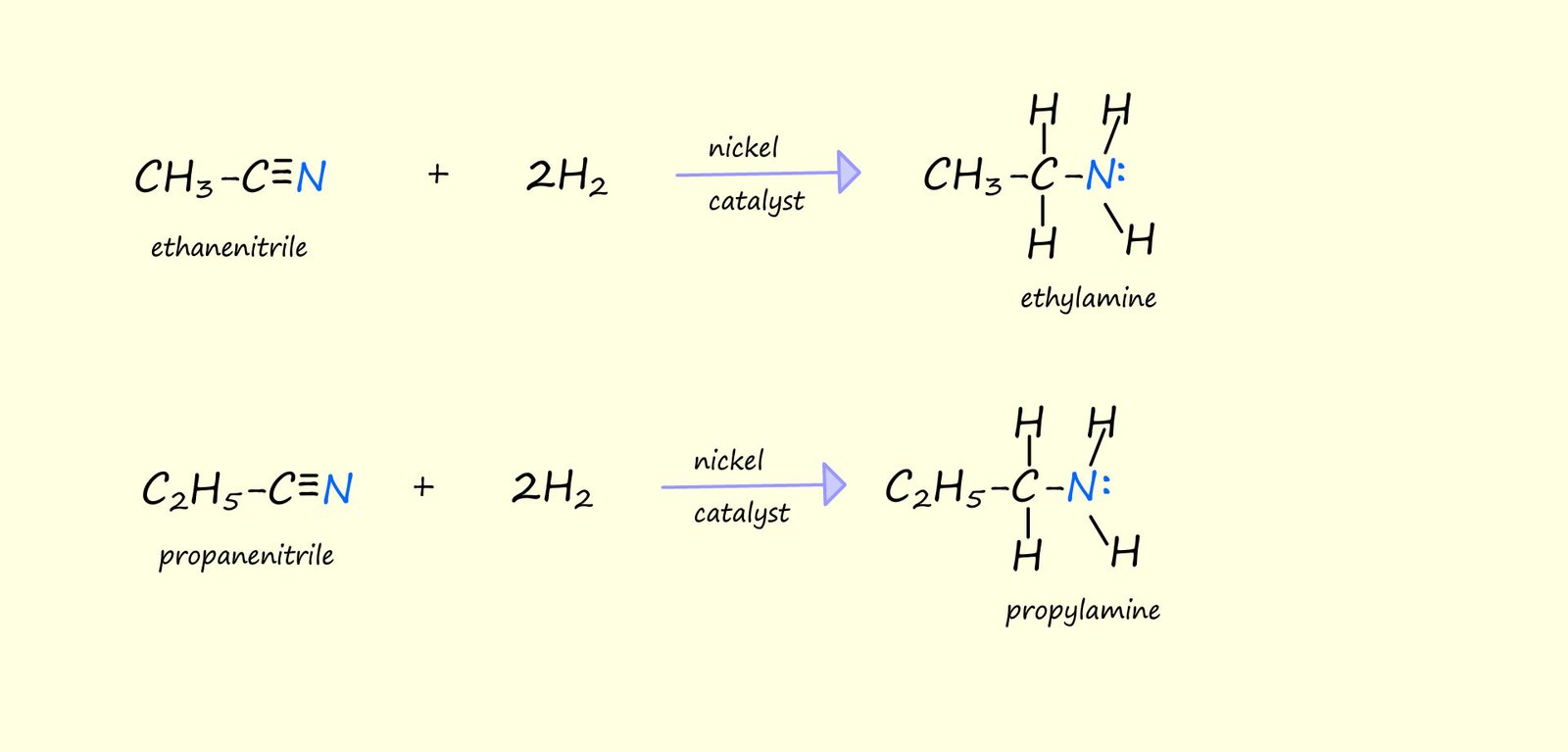
The nitrile group (R-CN) that can be reduced to form primary amines. This reduction can be carried in two ways:

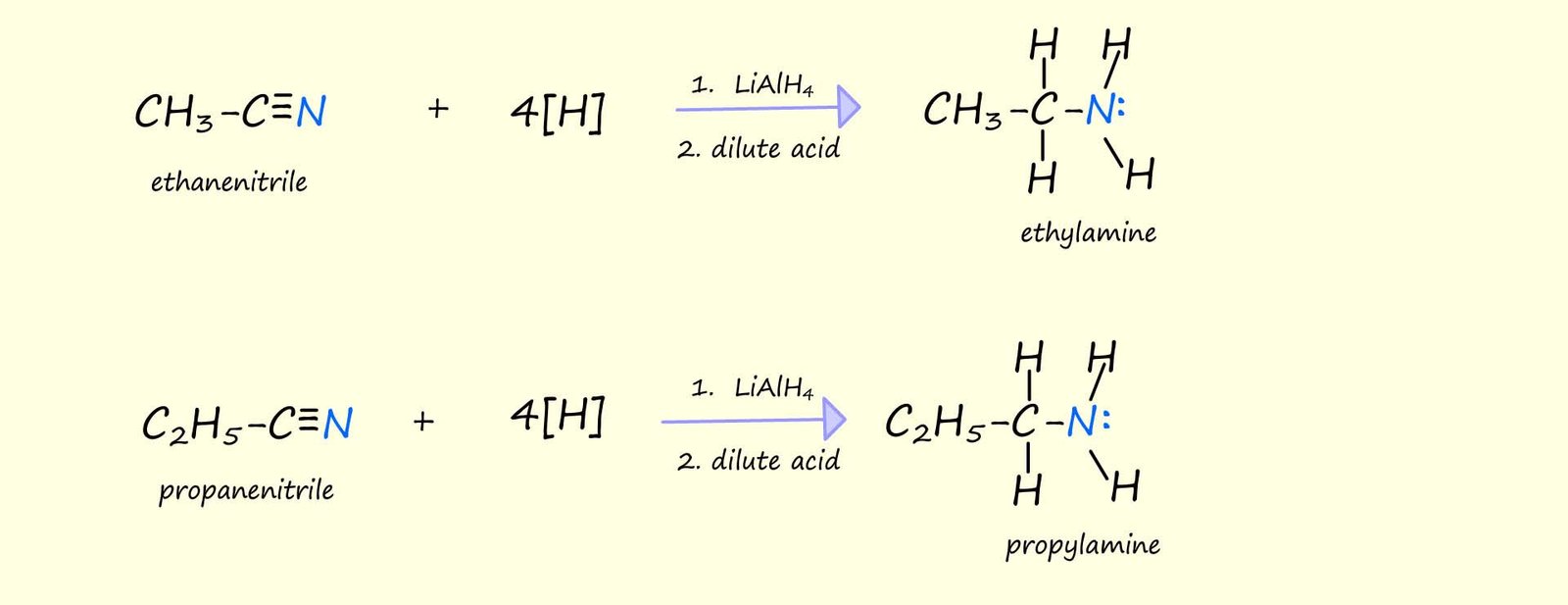
As an example consider the catalytic reduction of ethanenitrile and propanenitrile to form ethylamine and propylamine:
Nitriles can also be reduced to form primary amines using powerful reducing agents such as lithium aluminium hydride (lithium tetrahydridoaluminate, LiAlH4). This acts as a source of hydride ions (H-) which can attack the partially positively charged carbon atom (δ+) in the nitrile group. The reaction is carried out in dry ether as a solvent since LiAlH4 reacts very violently with water. To obtain the primary amine; the product of this reduction reaction dilute acid is added. The reaction can be written as shown below, where [H] represents the hydride ions (H-) produced by the lithium aluminium hydride.
 Primary aromatic amines can be produced by the reduction of the nitro group (-NO2) attached to an aromatic ring. The reducing agent used here is a mixture of tin or iron in concentrated hydrochloric acid; this reduction is carried using reflux conditions; as shown in the image opposite.
Primary aromatic amines can be produced by the reduction of the nitro group (-NO2) attached to an aromatic ring. The reducing agent used here is a mixture of tin or iron in concentrated hydrochloric acid; this reduction is carried using reflux conditions; as shown in the image opposite.
The diagram below outlines a route to produce the aryl amine aniline (phenylamine) from benzene:
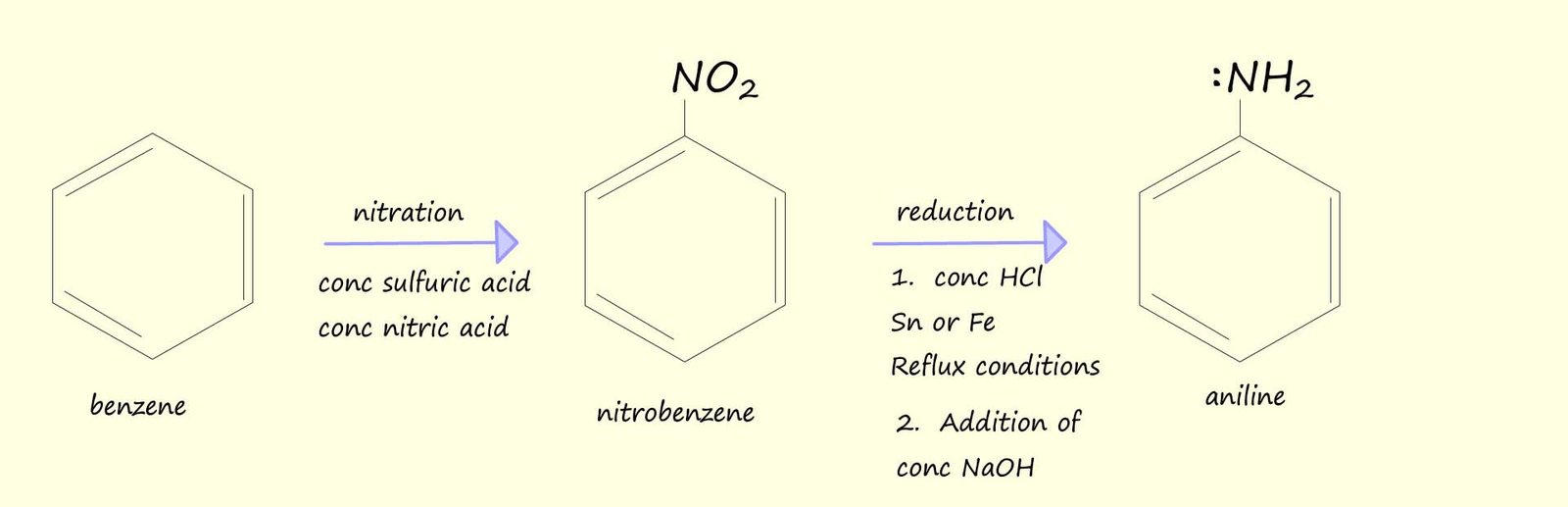
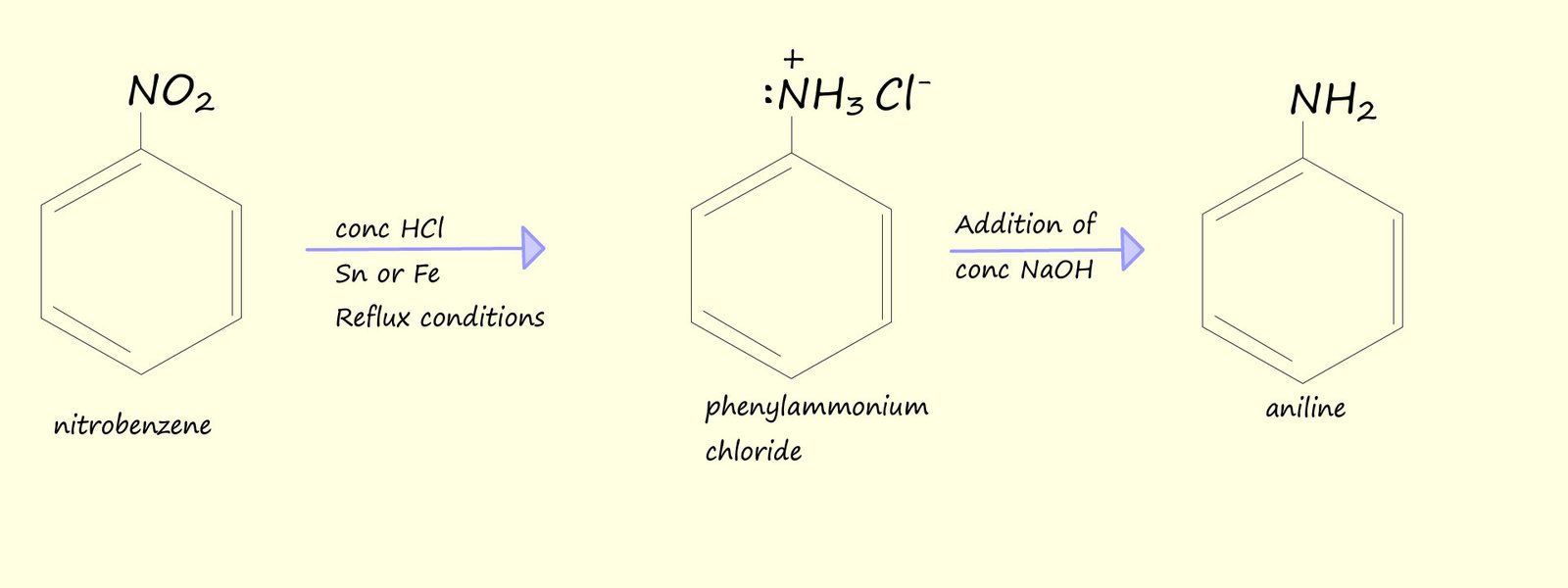
The nitration of aromatic rings is a reaction you are probably familiar with; the aromatic ring to be nitrated is refluxed with 3:1 mixture of concentrated sulfuric and nitric acid to form the aromatic nitro compound. Now the reduction of the nitro group (-NO2) into the basic amine group (-NH2) using concentrated hydrochloric acid and tin or iron will obviously result in the basic amino group reacting with the concentrated acid in an acid-base reaction to form a salt and water. This is outlined in the image below where nitrobenzene forms the soluble salt phenylammonium chloride. However addition of a strong alkali such as sodium hydroxide will result in the formation of the aryl amine aniline (phenylamine):
An overall equation to show the reduction of nitrobenzene into aniline is:
Here [H] is used to represent the reducing agent.
