


The halogenalkanes
or haloalkanes form a homologous series of organic compounds with the general formula
CnH2n+1X; where X is one of the halogens
(F, Cl, Br or I). The Haloalkanes have various everyday uses, such as in refrigerants in air conditioners and refrigerators, fire extinguishers (halons for fire suppression) and as solvents (e.g. chloroform in cleaning agents). They also play a role in the synthesis of many pharmaceuticals and pesticides. However due to many environmental concerns like ozone depletion many halogenalkanes (especially CFCs and halons, which used to be found in fire extinguishers) have been phased out and replaced by more environmentally friendly compounds.
Before we start looking at these nucleophilic substitution reactions a quick recap on what a nucleophile is:
Think of the halogenalkanes (or haloalkanes) as the ultimate starting material. On their own they might just be solvents or refrigerants but to a chemist they are an open door. Why? It all comes down to the carbon-halogen (C-X) bond; because the halogens are electronegative or electron-greedy elements; the C-X bond in a haloalkane is a polar bond with the carbon atom bearing a slight
positive charge (δ+) and
the halogen having a slight negative charge (δ-).
The
partial positive charge on the carbon atom attached to the
halogen makes it vulnerable to attack by electron rich species; that is
nucleophiles.
The (δ+) charge on the carbon atom in the C-X bond is like a huge open invitation for
nucleophiles to come charging in and knock out the
halogen atom. The C-X bond is already a polar one due to the fact that the
halogens are electronegative elements and they are also good leaving groups simply because they can form stable halide ions. So really the stage is set; all it needs is a suitable
nucleophile to come in and kick out or replace the
halogen atom.
Curly arrows are not decoration — they are your electron story. Get them right and you’re already on the examiner’s good side 🙂
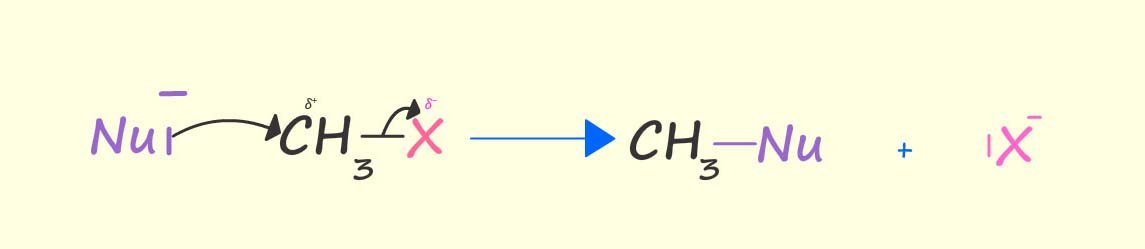
In the diagram shown below a nucleophile (Nu-)
uses its lone pair of electrons to form a new
covalent bond to the
carbon atom attached directly to the bromine atom (the halogen) in a molecule of bromomethane and at the same time
the carbon-bromine bond breaks and a bromide ion (Br-) leaves, for this reason it is often referred to as the leaving group. The end result
is that the bromine atom is replaced or
substituted by the nucleophile; this is an example of a nucleophilic substitution reaction.
Now the two most common reaction types that the haloalkanes readily undergo are nucleophilic substitutions and elimination reactions. This page will cover only nucleophilic substitution reactions; click the link above if you would like to visit the page on elimination reactions.
In a nucleophilic substitution reaction the halogen atom is replaced or substituted with another atom or group for example:
The way in which a haloalkanes react with a nucleophile can in part be down to their structure; that is whether they are primary, secondary or tertiary haloalkanes. So let us start by looking at some structural isomerism involving haloalkanes.
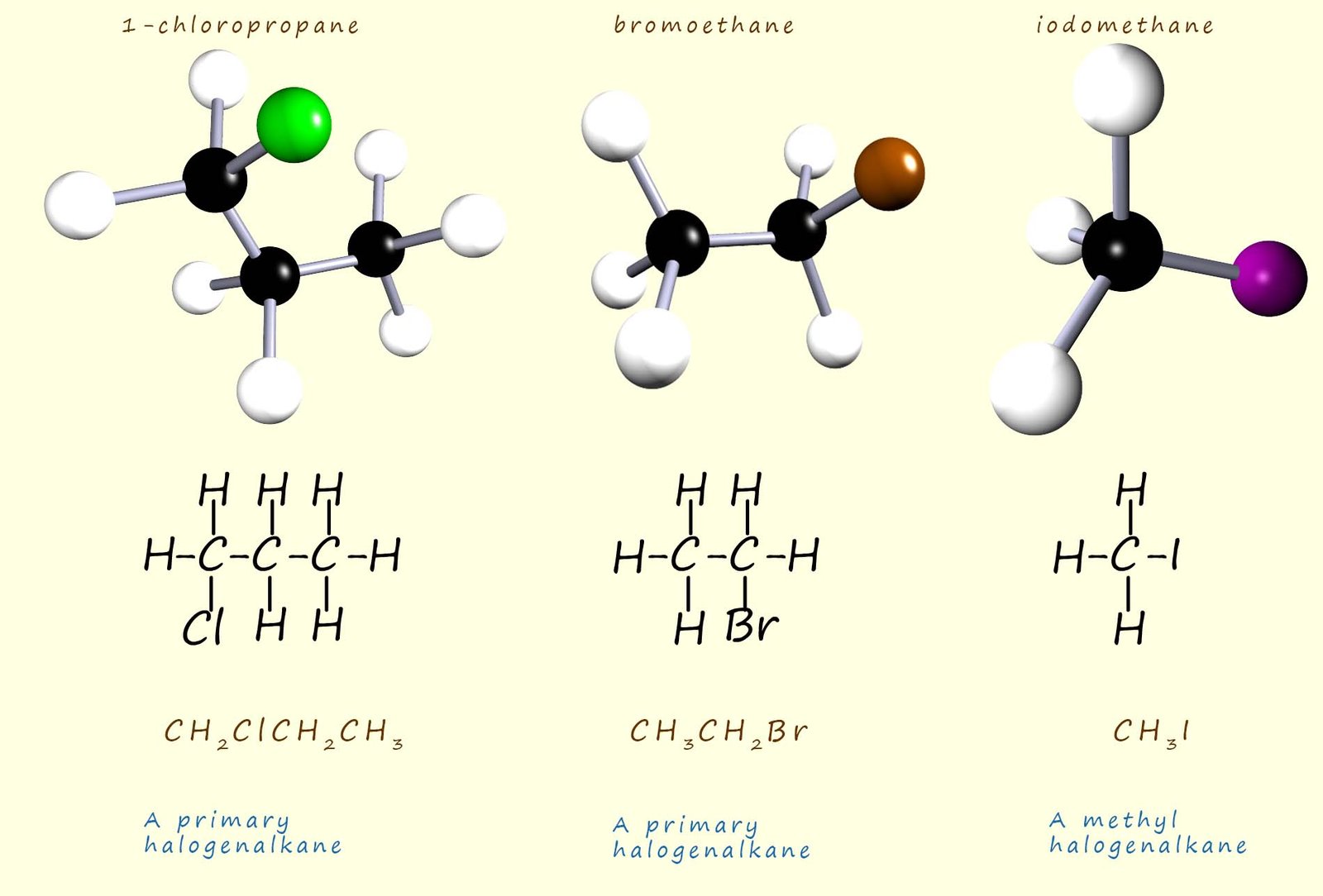
The image below shows two primary haloalkane molecules. In a primary halogenalkane molecule the carbon atom attached to the halogen atom (X) will have one alkyl group (-R) and two hydrogen atoms attached to it. Whereas the last molecule in the image below is simply a methane molecule where one of the hydrogen atoms has been replaced by a halogen, in this case an iodine atom. Simple haloalkane molecules such as this where the carbon atom attached to the halogen is bonded to only hydrogen atoms are called methyl haloalkanes.
In a secondary haloalkane molecule the carbon atom joined directly to the halogen will have two alkyl groups (-R) and one hydrogen atom attached to it; two examples of secondary haloalkane molecules are shown below:

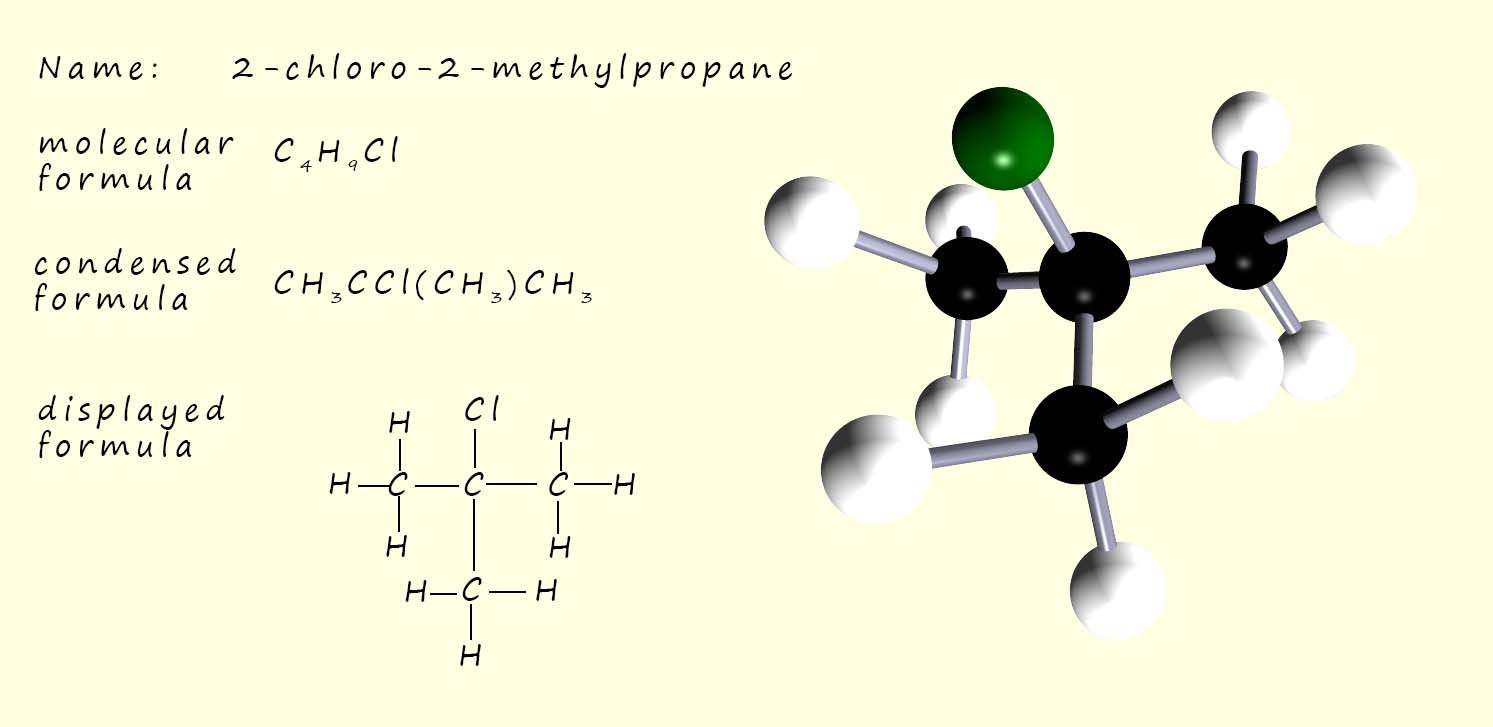
The image opposite shows 2-chloro-2-methylpropane, a tertiary haloalkane. In a tertiary haloalkane molecule the carbon atom attached to the halogen has three alkyl groups (-R) directly attached to it; this means that there are no hydrogen atoms attached.
The halogens being electronegative elements means that the C-X bond in haloalkane molecules will be a polar one, with the carbon atom in the C-X bond having a partial positive charge (δ+) and the halogen atom having a partial negative charge (δ-). A nucleophile is an electron rich species; nucleophiles can be neutral molecules or ions with lone pairs of electrons which they can donate to an electron deficient molecule/atom (electrophiles); such as the carbon atom in a polar C-X bond. Examples of common nucleophiles were mentioned above but include hydroxide ions (OH-), cyanide ions (CN-), ammonia (NH3) and water (H2O). All these molecules whether charged or neutral are able to act as nucleophiles simply because they have lone pairs of electrons.
Complete the quick activity below to correctly identify whether the molecule is a primary, secondary or tertiary haloalkane molecule.
Rule: look at the carbon attached to X. Count how many alkyl groups are attached to that carbon: 1 = primary, 2 = secondary, 3 = tertiary.
Note
The nucleophilic substitution reactions that haloalkanes undergo can follow one of two different mechanisms or routes. These mechanisms are called SN1 and SN2; this is short for substitution nucleophilic unimolecular (SN1) and substitution nucleophilic bimolecular (SN2). The mechanism, either the SN1 or SN2 route that is followed by the haloalkane when it reacts with a nucleophile will depend on whether it is a primary, secondary or tertiary haloalkane.
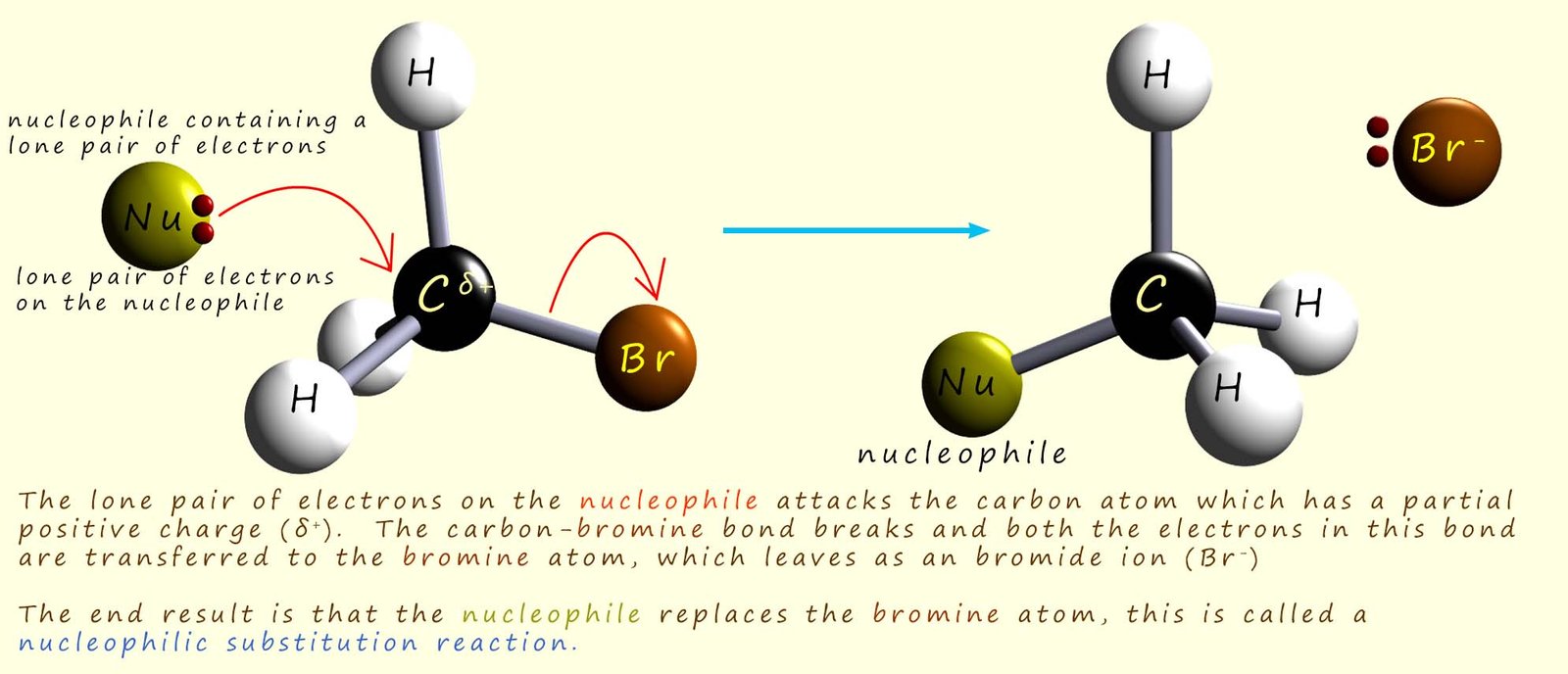
The image below shows the hydroxide ion (OH-) using one of its lone pairs of electrons to attack the δ+ carbon atom in a bromomethane molecule to form the alcohol methanol and a bromide ion (Br-). Now at any given temperature and concentration of the two reactants, the hydroxide ion and the bromomethane; the reaction occurs at a certain rate. If we were to double the concentration of the hydroxide ion then the rate of reaction would also double. Similarly if we double the concentration of the bromomethane then the rate of reaction also doubles. This tells us that the rate of reaction depends on the concentration of both starting reagents; that is it is a second order reaction. The rate of the reaction will change if we alter the concentration of either the hydroxide ion or the bromomethane.

The mechanism put forward to explain this observation is called the SN2 mechanism or substitution nucleophilic bimolecular. The main features of this mechanism are outlined in the image below and can be broken down into a number of steps:

There are a number of factors that affect how quickly these SN2 reactions proceed; one of the most obvious factors is anything which can block or at least slow or hinder the incoming nucleophile as it attacks the δ+ carbon atom in the C-X bond will obviously affect how quickly this reaction can take place. Since the incoming nucleophile (Nu) needs to approach the δ+ carbon atom from the side opposite to the leaving group (often described as a 180° approach), then any large bulky groups attached to the carbon atom will could slow its approach or even block it. This blocking action of the attached groups is often referred to as steric hindrance.
Steric hindrance refers to the effect of large, bulky groups crowding around a reaction site and physically blocking or slowing down the approach of another molecule or ion.
In SN2 reactions steric hindrance reduces the rate of reaction because the nucleophile must approach the δ+ carbon atom from behind at an angle of 180° and bulky alkyl groups get in the way; making this backside attack more difficult or even impossible.
In the example shown in the image above the δ+ carbon atom in the C-X bond is bonded to three very small hydrogen atoms; so what do you think would happen to the rate of the reaction if one of these hydrogen atoms was replaced by a larger group such as a methyl group (-CH3) or an ethyl group (-C2H5) or even larger groups? As you might expect as the groups become larger and more of them are added they do indeed block the incoming nucleophile and slow the reaction rate.

This is summarised in the diagram below which starts with a methyl haloalkane molecule which undergoes a rapid SN2 reaction and the final image shows a tertiary haloalkane molecule which does not undergo an SN2 reaction but instead reacts via a SN1 reaction mechanism.
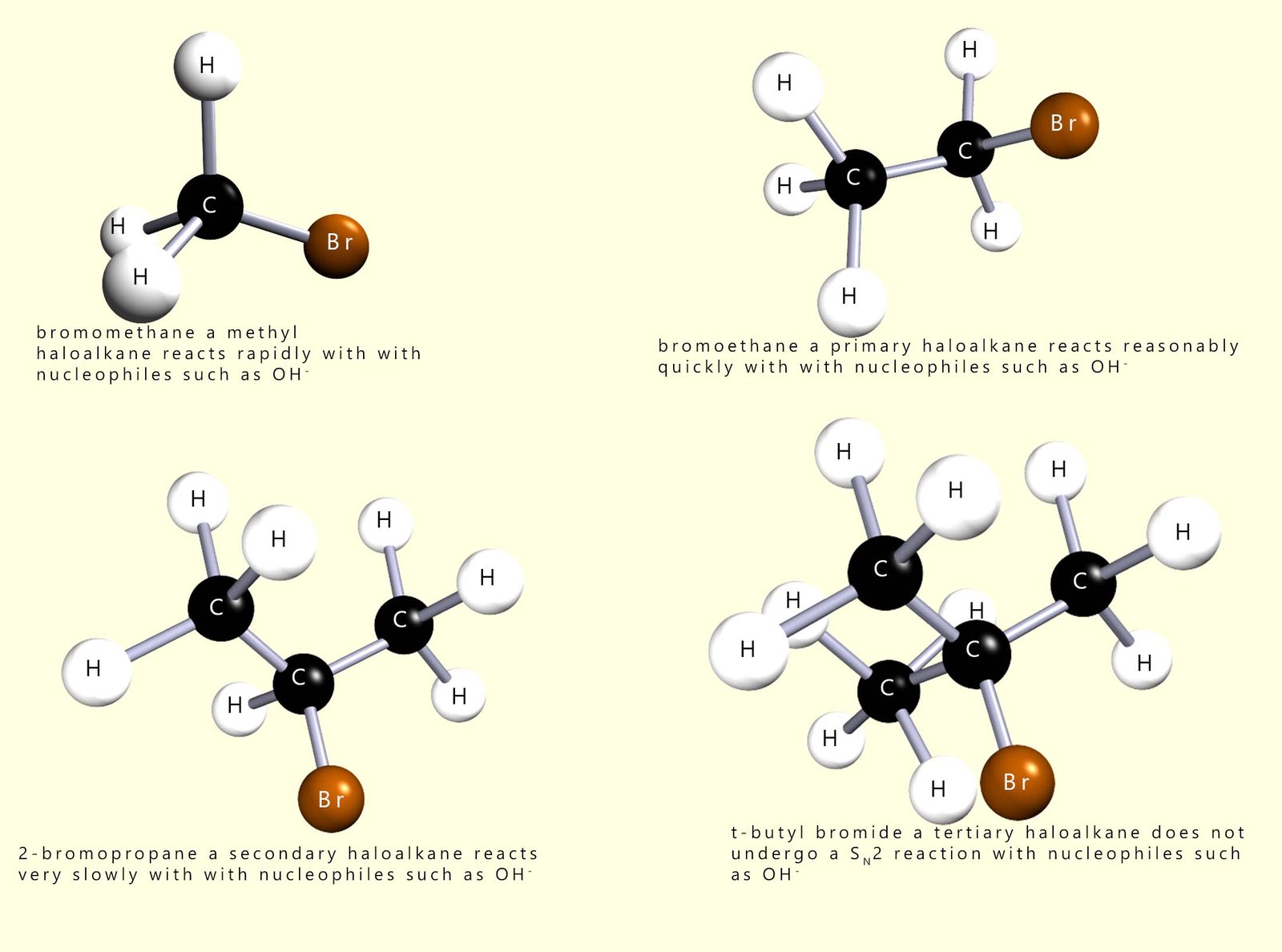
Nucleophilic substitution reactions involving primary haloalkanes readily take place by an SN2 mechanism; since the incoming nucleophile is not blocked or hindered in any way from attacking the δ+ carbon atom; however as the size of the groups attached to the carbon atom bonded to the halogen increase the rate of reaction slows down because these larger groups block the incoming nucleophile from approaching the δ+ carbon atom effectively.
Now consider secondary haloalkanes which have two alkyl groups attached to the carbon bonded to the halogen; these groups are more effective at blocking the incoming nucleophile and so secondary haloalkanes react more slowly than primary halogenalkanes. So what about tertiary haloalkanes? You will probably have figured out that tertiary haloalkanes do not react with nucleophiles by an SN2 mechanism, the incoming nucleophile is simply blocked by the three alkyl groups. We can summarise this as shown below:
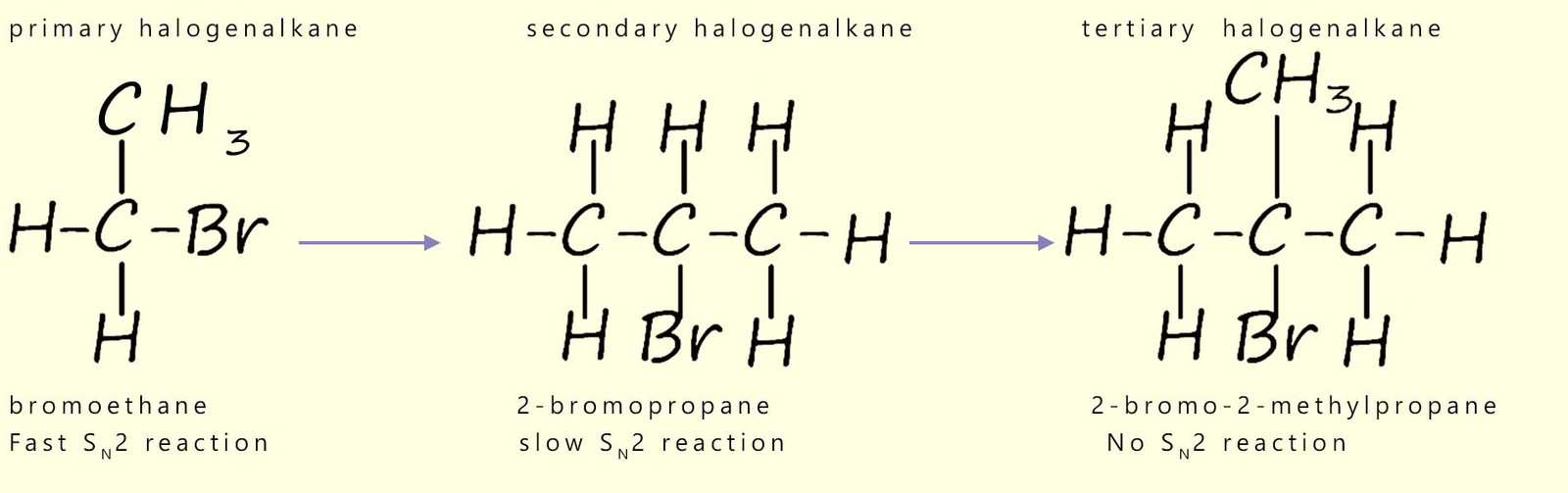
You do not get marks just for naming a mechanism. You get marks for showing you understand what is happening.
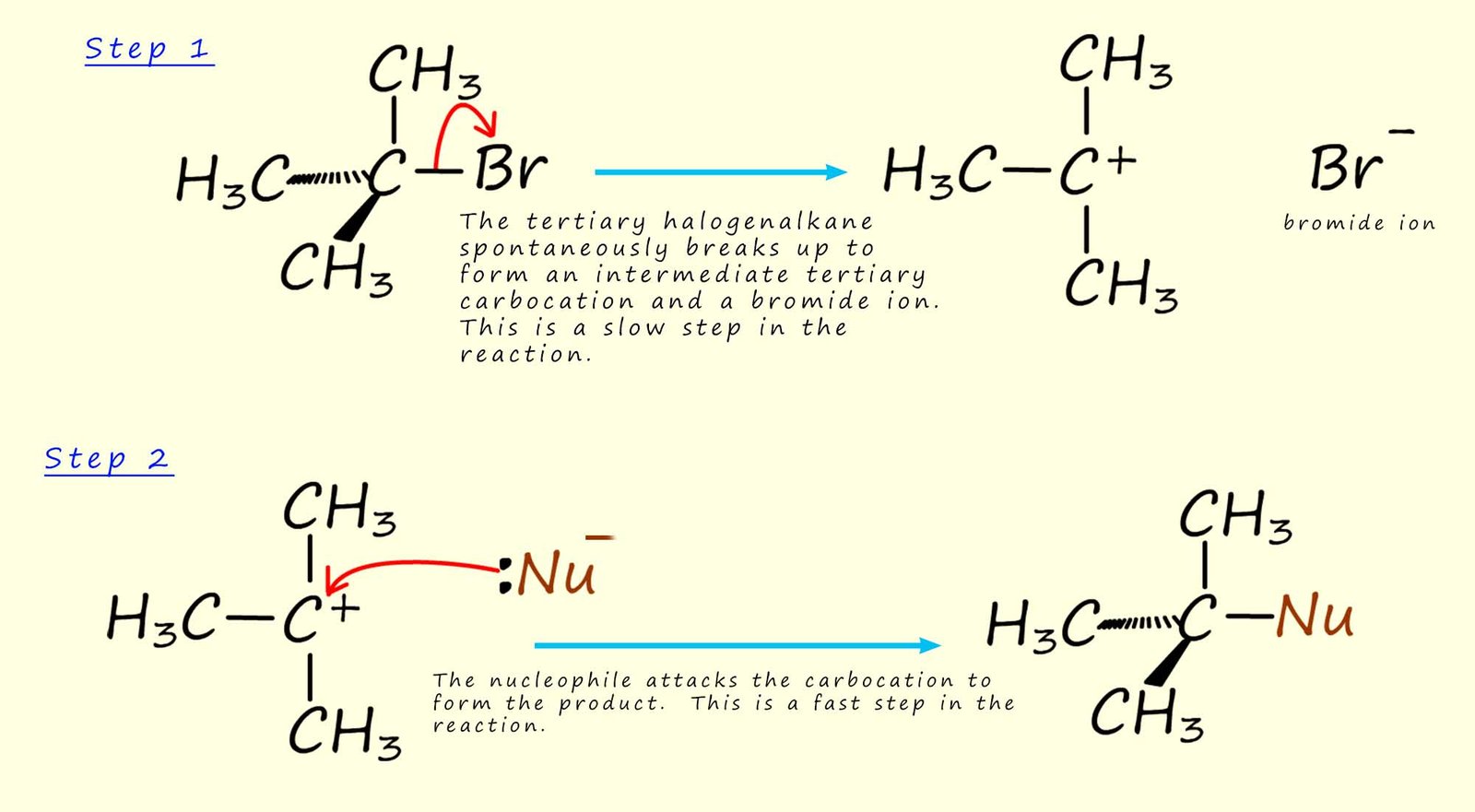
So how do tertiary haloalkanes react with nucleophiles? The mechanism suggested for these reactions involving tertiary haloalkanes is called an SN1 reaction ( substitution nucleophilic unimolecular).
For example if we use 2-bromo-2-methylpropane as an example of a tertiary haloalkane (as shown in the image above), then in its reaction with a nucleophile the rate of reaction depends only on the concentration of the haloalkane and not the nucleophile. This is different from the SN2 mechanism where the reaction rate depends on both the concentration of the nucleophile and the haloalkane. The mechanism for the SN1 is outlined below. The reaction of tertiary haloalkanes via this SN1 mechanism occurs in two steps:
Since step 1 is the slow step, this holds up the reaction. Step 2 can be very fast, but it makes no difference to the overall rate of the reaction; this depends only on step 1 (the rate limiting step).
If primary haloalkanes molecules are the masters of SN2 nucleophilic substitution reactions and tertiary haloalkane molecules are the kings of SN1 reactions then where does that leave secondary haloalkanes?
The positive inductive effect (+I) refers to the ability of alkyl groups to push electron density through σ bonds towards a positively charged or electron-deficient carbon atom.
In haloalkanes the alkyl groups attached to the carbon bonded to the halogen help to stabilise a positive charge. This is why tertiary carbocations are more stable than secondary, which are more stable than primary carbocations. 🔋
This stabilisation is especially important in SN1 reactions, where formation of a carbocation intermediate is the slow, rate-determining step. 🚦
Secondary haloalkane molecules are like the annoying "middle children" of organic chemistry, in that they can actually undergo both SN2 and SN1 mechanisms. So how is this possible? Well they have two alkyl groups so they have a bit of steric hindrance (blocking the SN2 attack) but they also a bit of inductive stabilisation from the two alkyl groups which helps stabilise the intermediate carbocation in the SN1 reaction. So how does the secondary haloalkane molecule decide which path to take- SN2 or SN1? Well it comes down to a battle of conditions.
When a secondary halogenalkane is sitting on the fence trying to decide whether to follow a SN2 or SN1 route, the environment and the type of nucleophile tips the scale. The reaction will follow a:
However, there is another factor you need to be aware of before deciding whether a reaction follows an SN2 or an SN1 pathway, this factor is the type of solvent used to carry out the reaction in, that is whether it is a aprotic or protic solvent.
An aprotic solvent is one that lacks hydrogen atoms bonded to electronegative atoms such as oxygen or nitrogen, this prevents strong hydrogen-bonding interactions with any nucleophiles, so then nucleophiles remain more reactive, favouring SN2 reactions. Common aprotic solvents used in SN2 reactions include acetone or propanone (CH3COCH3), dimethyl sulfoxide ((CH3)2SO), and acetonitrile (CH3CN).
In contrast protic solvents form hydrogen bonds with nucleophiles, reducing their nucleophilicity and slowing SN2 reactions. Protic solvents favour SN1 reactions because they help stabilise the carbocation intermediate formed in the rate-determining step. Examples of protic solvents include water and alcohols such as methanol (CH3OH) and ethanol (C2H5OH).
Welcome to the world of organic synthesis! Haloalkanes are the “Lego bricks” of chemistry. Before you learn any labels, can you read the situation? Pick the crowd level at the carbon attached to X, then pick what that suggests.
There are two other factors that are important when discussing the rates of SN1 and SN2 reactions, these are:
As you move down group 7 from the very electronegative fluorine to the much less electronegative iodine, the polarity of the carbon-halogen bond decreases and so the electron deficient carbon atom is less strongly δ+.
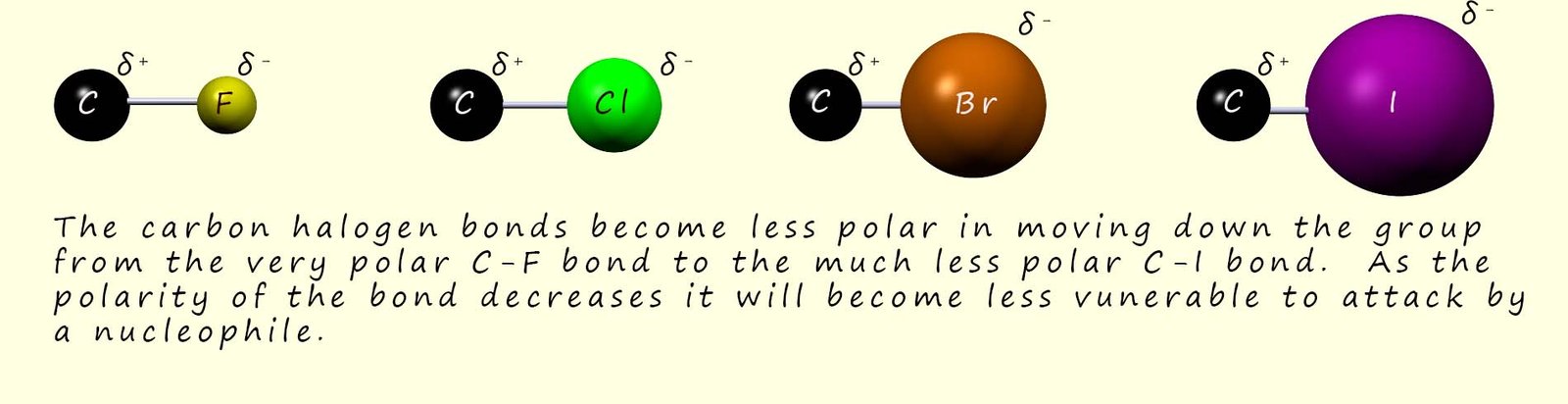
Based on bond polarity alone you might predict fluoroalkanes would react more readily than iodoalkanes, since the incoming nucleophile would be more strongly attracted to the larger partial positive charge on the carbon atom; however in practice fluoroalkanes react extremely slowly in nucleophilic substitution reactions because the C-F bond is very strong (see the image below), so if the halogen won't leave the nucleophile cannot come in and bond to the carbon atom in the C-X bond. However the C-I bond is the weakest of the carbon halogen bonds and this is why iodoalkanes are generally the most reactive overall.
The general trend in the strength of the C-X bond is that bond strength decreases as you move from F to Cl to Br to I. This is mainly because the bond length increases due to the larger atomic size of the halogen, and longer bonds generally mean weaker bonds, as outlined in the image below:

Of the two factors, it is the strength of the C-X bond (bond enthalpy) rather than bond polarity that has the major effect on the overall rate of reaction of haloalkanes, and we can say that the rates of reaction of haloalkanes follow the pattern:
iodoalkanes > bromoalkanes > chloroalkanes > fluoroalkanes
| Feature 🧪 | SN2 reaction 🔄 | SN1 reaction 🧩 |
|---|---|---|
| Type of halogenalkane | Primary (and methyl; some secondary) | Tertiary (and some secondary) |
| Mechanism steps | One step | Two steps |
| Intermediate formed | No intermediate Transition state only |
Carbocation intermediate |
| Rate depends on | Halogenalkane and nucleophile | Halogenalkane only |
| Order of reaction | Second order | First order |
| Steric hindrance 🚧 | Strong effect Bulky groups slow reaction |
Much less important in the rate-determining step (bond breaking). Crowding may affect nucleophile capture. |
| Typical solvent | Polar aprotic solvent | Polar protic solvent |
Read and review the 7 statements below, they are there o help you avoid these common pitfalls in answering exam questions. Why not make a set of flash cards using these statements!
Before you move on, can you tick all of these?