

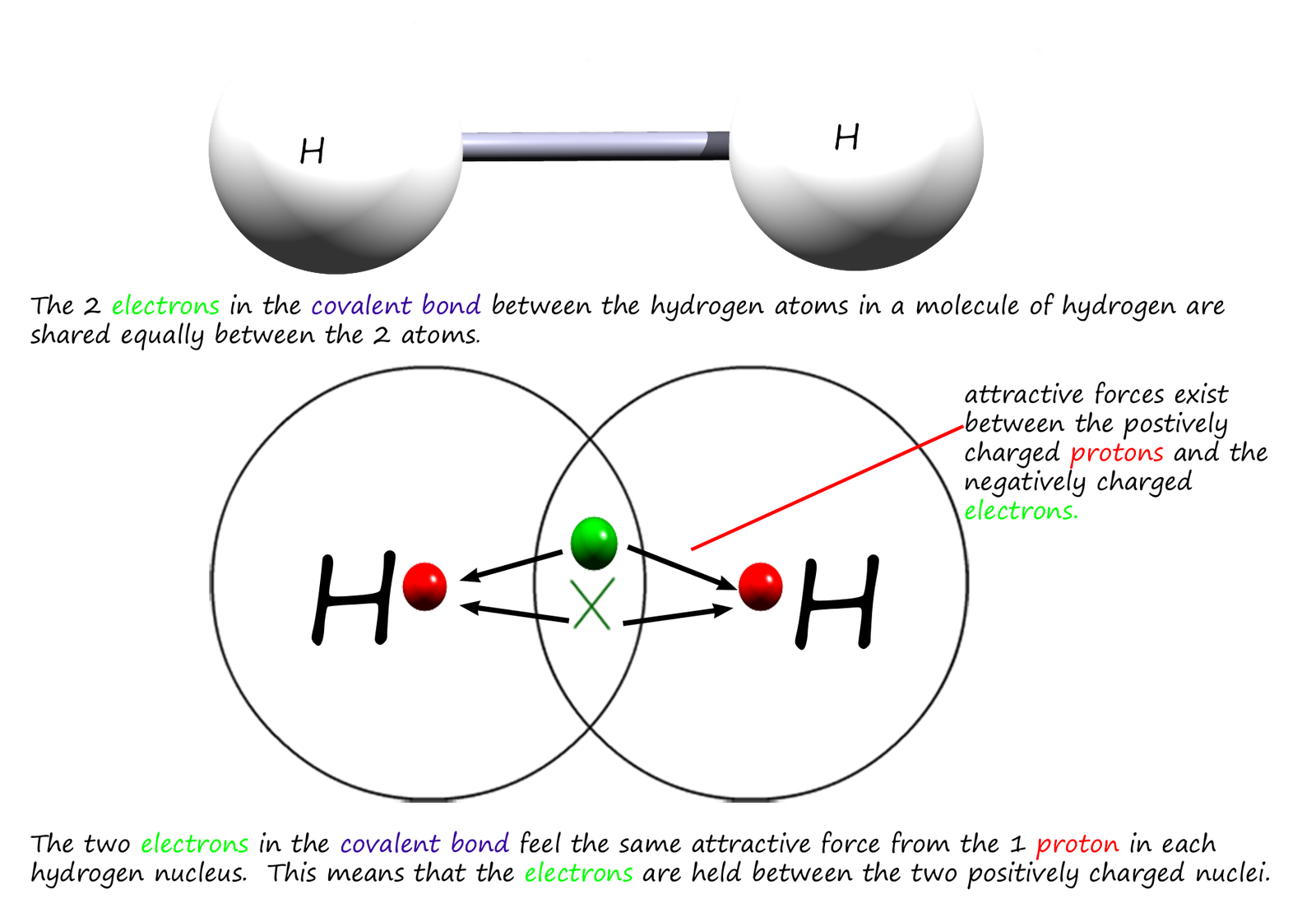
A covalent bond is usually formed when 2 non-metals atoms join and share a pair of electrons EQUALLY between them. The diagram below shows a covalent bond formed between 2 atoms of hydrogen, you should recall that the two electrons in the covalent bond between the two hydrogen atoms are attracted to each of the positively charged hydrogen nuclei. The two negatively charged electrons will feel the same attractive force from each nucleus since both nuclei contain 1 positively charged proton. This means that that the two electrons are shared equally between the two hydrogen atoms. You will no doubt have learned from your gcse course that a covalent bond involves the equal sharing of a pair of electrons. This is outlined in the diagram below:
However what happens if the molecule contains different atoms? Will the electrons in the covalent bond still be shared equally this time? Consider as an example a molecule of hydrogen fluoride.
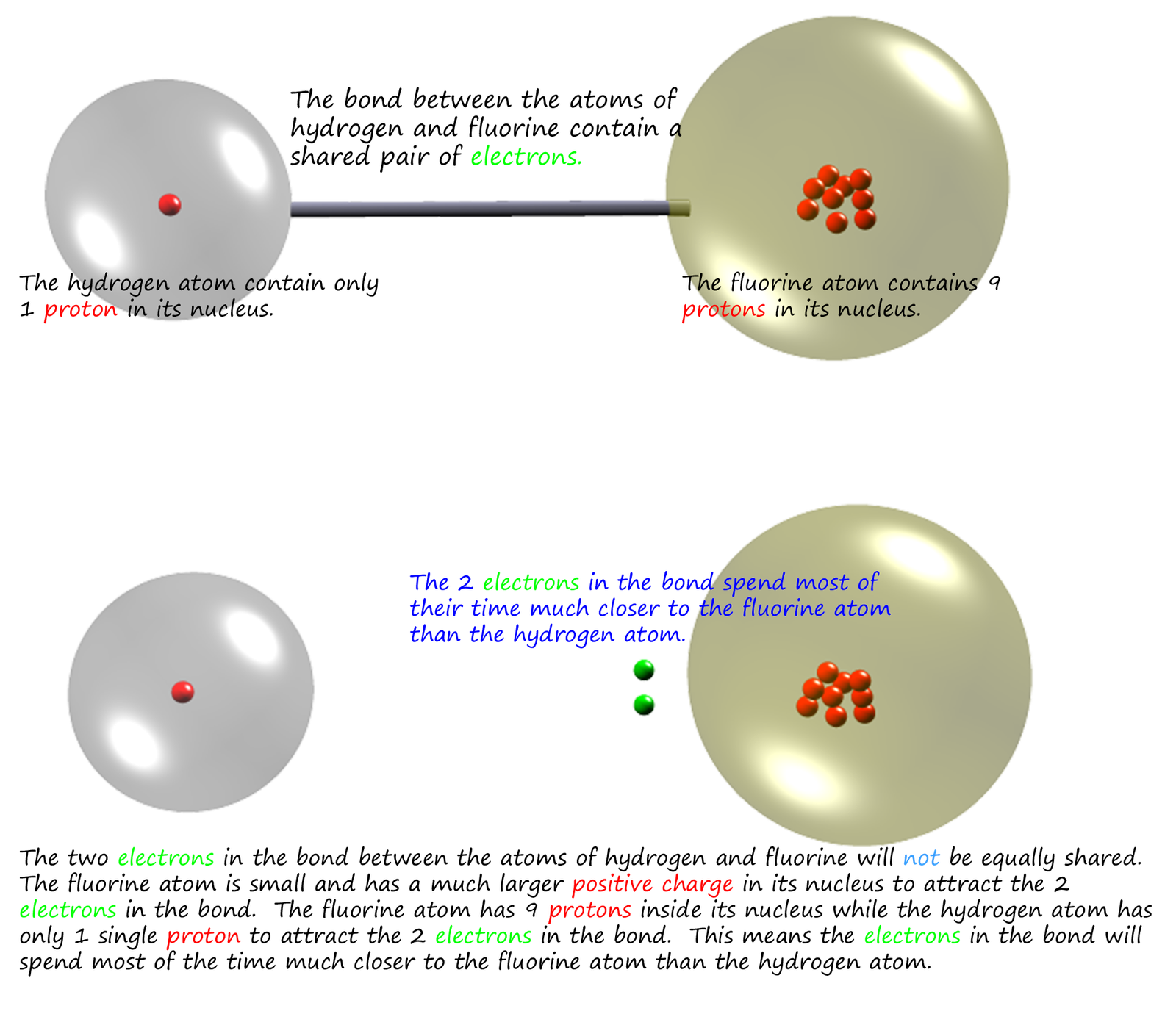
The fluorine atom has a much larger nuclear charge (+9) compared the (+1) nuclear charge
in the hydrogen nucleus, so the fluorine atom will be more able to attract the electrons in the
covalent bond than hydrogen atom; we say that fluorine
atom is
more electronegative than the hydrogen atom. Electronegativity
is the ability of an atom in a covalent bond to attract
electron density towards it.
 The Electronegativity of an element depends on a number of factors. One factor is the
effective nuclear charge
that the electrons in the outer valency shell feel, since these are the electrons involved in actually forming covalent bonds. For example fluorine has an
electron arrangement of 2,7 or an electron configuration of 1s22s22p5.
The two inner shell electrons in the 1s sub-shell
will screen or shield two protons from the outer shell electrons and since
electrons in the same shell are not particularly
effective at shielding each other this means that the outer valency shell
electrons will feel an effective nuclear charge of 9-2=+7.
The Electronegativity of an element depends on a number of factors. One factor is the
effective nuclear charge
that the electrons in the outer valency shell feel, since these are the electrons involved in actually forming covalent bonds. For example fluorine has an
electron arrangement of 2,7 or an electron configuration of 1s22s22p5.
The two inner shell electrons in the 1s sub-shell
will screen or shield two protons from the outer shell electrons and since
electrons in the same shell are not particularly
effective at shielding each other this means that the outer valency shell
electrons will feel an effective nuclear charge of 9-2=+7.
For
hydrogen the effective nuclear charge is only +1 since it contains only the 1 proton and 1 electron. Although the method used above to work out the effective nuclear charge is rather crude it at least gives a rough working guideline, if you wish to learn more about how to calculate effective nuclear charge more accurately then a quick internet search on Slater values will no doubt point you in the right direction, though calculating Slater values is relatively straightforward it is not required at A-level.
As we move across a period in the periodic table, the number of inner shell electrons remains constant, but the nuclear charge increases. This increase in nuclear charge pulls the electrons closer to the nucleus, resulting in smaller atomic radii. Consequently, electronegativity increases across a period because the atoms become more effective at attracting electrons in a covalent bond.
The size of the atom will also have an effect on its electronegativity value. As we descend a group in the periodic
table the effective nuclear charge remains the same but the atoms obviously get larger; this means that the
nucleus is further away from any electrons in a covalent bond and so will be less able to attract any
electron density in a covalent bond. This means that the electronegativity
decreases down a group e.g.
consider the two alkali metals lithium and potassium as shown in the image below.
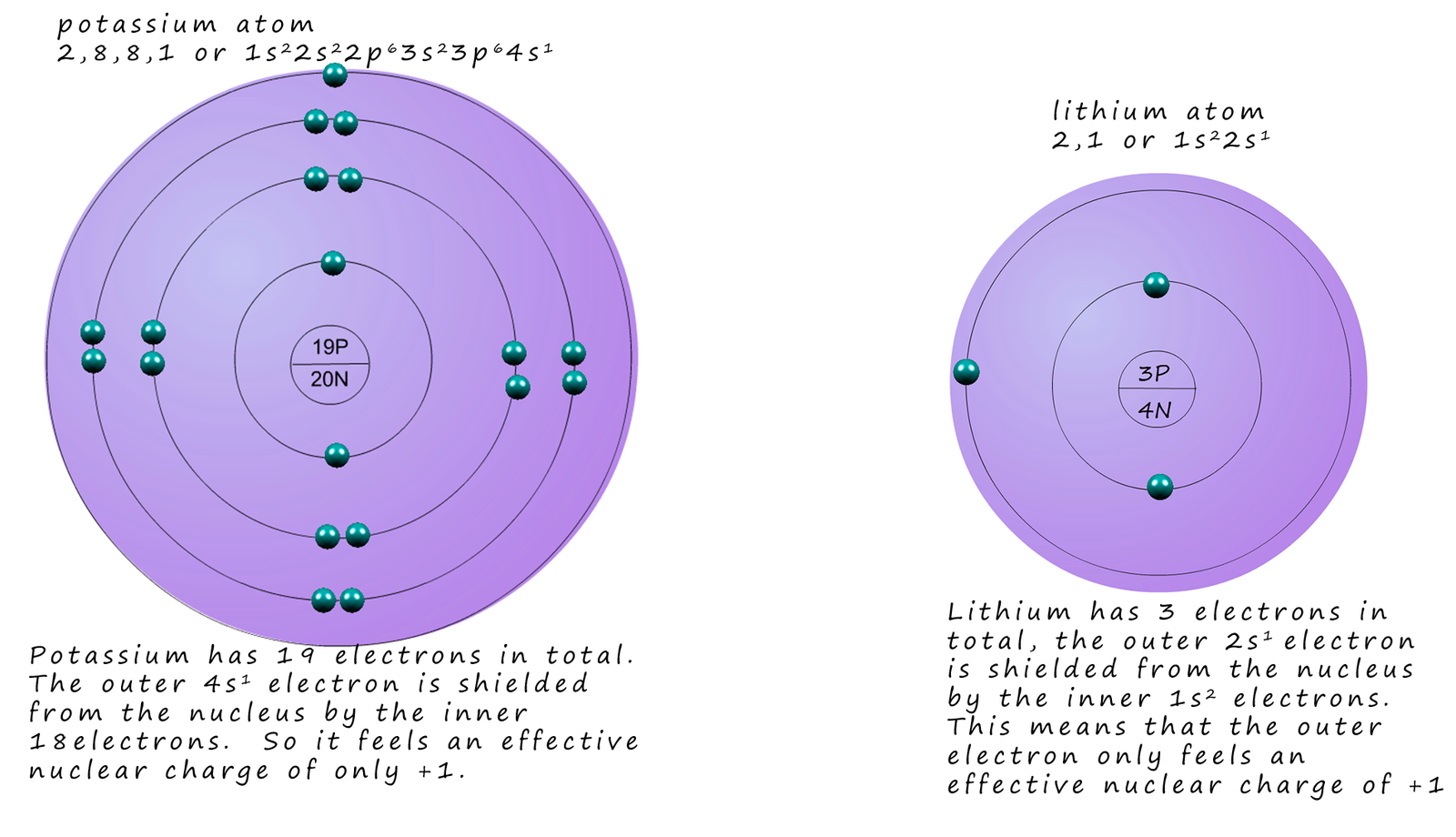
Potassium atoms (19K) are obviously larger than lithium atoms (3Li) and also have a
larger nuclear charge (+19)
however there are 18 electrons between the nucleus
and the outer 4s1 electron. These 18 electrons
will
effectively shield or screen out 18 protons or 18 positive charges.
This means that the outer 4s1 electrons will only feel an
effective nuclear charge of +1 and NOT +19.
A similar argument can be made for lithium; the inner 1s2 electrons will shield or "cancel" out the positive charge from 2 protons in the nucleus; so as with potassium the outer 2s1 electron in lithium will feel an effective nuclear charge of +1. However the outer electron in lithium is closer to the nuclear charge so it will feel a greater attraction from the effective +1 nuclear charge. The outer valency electron in potassium is much further from the nucleus so the attraction to the +1 effective nuclear charge this time will be less, this is shown in the diagram above and summarised in the table below.
| Metal | Electron arrangement | Number of shielding electrons (all electrons except those in the last shell) | Effective nuclear charge |
|---|---|---|---|
| Lithium | 1s22s1 | 2 | +1 |
| Potassium | 1s22s22p63s23p64s1 | 18 | +1 |
The trends in the electronegativity values in a group and across a period in the periodic table as shown in the image below:
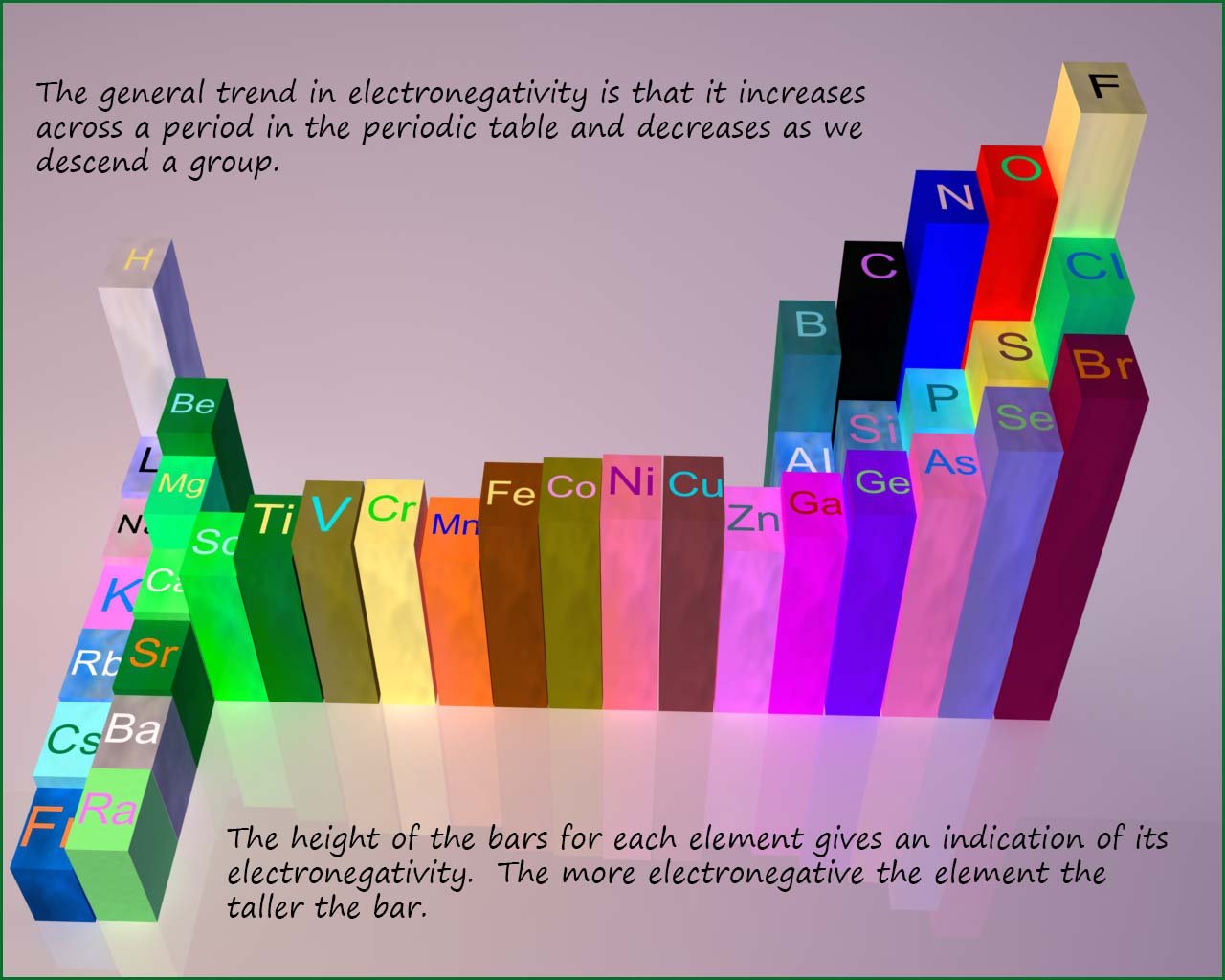
Small atoms with large effective nuclear charges
have large electronegativity values. The most
electronegative element
in the periodic table is fluorine while the least electronegative
element is francium. The image above illustrates
the trends in electronegativity across the periodic table.
As we descend a group the atomic radius of the atom
increases while the effective nuclear charge remains constant;
this means that electronegativity
decreases down a
group in the periodic table. As we cross a period in the periodic table the atomic radius
decreases while the
effective nuclear charge increases; this means that the electronegativity increases across a period. The alkali
metals in group 1 of the periodic table have low electronegativity values whereas the halogens in group 7 have
higher electronegativity values.
Electron affinity is the ability of an isolated atom to attract an electron
and the ionisation energy is a measure of how easy
or hard it is to remove an electron from an atom. So one of the ways to measure
electronegativity is to take
an average of the electron affinity and the ionisation energy. A scale can be produced from this and a number assigned
to each element to represent its electronegativity (note the scale or number has no units). On this scale the
most electronegative element fluorine is assigned a value of 4 and all other elements
electronegativity values
are measured relative to this. The table below lists some values of electronegativity for the elements you are
likely to meet. You can see that N, O and F are the most electronegative elements in the periodic table. It is also clear to see the trends in the
electronegativity values across a period and down a group in the periodic table.
It is worth mentioning that if you look online or in textbooks you may see slightly different
values of electronegativity for some elements; this is simply due to the way in which it is measured. However the
same patterns or trends should be obvious no matter which method is chosen.
| H 2.1 |
He | ||||||
| Li 1.0 |
Be 1.5 |
B 2.0 |
C 2.5 |
N 3.0 |
O 3.5 |
F 4.0 |
Ne |
| Na 0.9 |
Mg 1.3 |
Al 1.5 |
Si 1.9 |
P 2.2 |
S 2.6 |
Cl 3.0 |
Ar |
There are no values listed for the noble gases; this is simply because they rarely form covalent compounds and have no affinity for electrons.