Covalent bonding as you learned in your gcse chemistry lessons involves the sharing of a pair of electrons. The two atoms involved in forming a covalent bond are held together by the electrostatic attraction of the positively charged nuclei and the negative charges on the shared pair of electrons. Covalent bonding usually but not always occurs between non-metal elements, this is outlined in the image below:
Reactive non-metals are mostly found in groups 5, 6 and 7 of the periodic table so this means
that their outer electron shells are almost full; so when these non-metal elements react they will gain
electrons which will result in them having full outer electron shells or a noble gas
np6 electron configuration in their outer electron sub-shell.
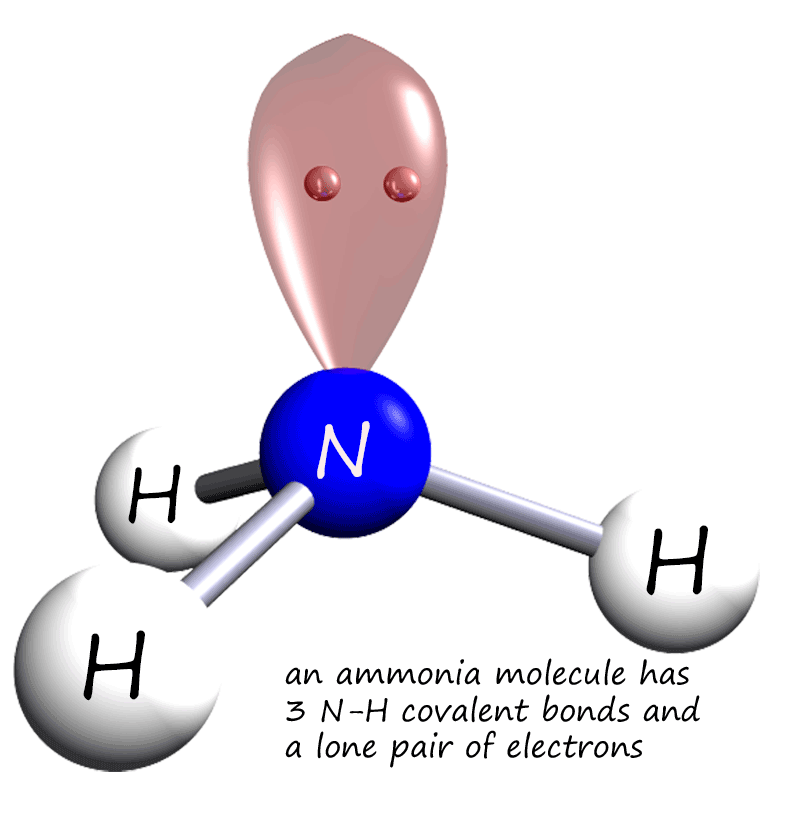 Ammonia is a small covalent molecule; the
ammonia molecule has 3N-H covalent bonds and a lone pair or
non-bonding pair of electrons. The image opposite shows an ammonia
molecule with its lone pair of electrons.
Ammonia is a small covalent molecule; the
ammonia molecule has 3N-H covalent bonds and a lone pair or
non-bonding pair of electrons. The image opposite shows an ammonia
molecule with its lone pair of electrons.
Below is a dot and cross diagram to show how each of the covalent bonds in an ammonia
molecule are formed. The nitrogen atom has an electron arrangement of 2,5 or an electron configuration of
1s22s22p3 this is shown below. Its outer
shell electrons are shown as green dots; now recall from gcse chemistry that in dot and cross diagrams we only show the
outer shell or valency electrons since these are the ones involved in forming bonds. Hydrogen is also shown with its one outer shell
electron; shown as a black cross (X).
The octet rule can be useful here in drawing the dot and cross diagram for the ammonia molecule. In order to achieve full last electron shells both these non-metal atoms share electrons. The
electrons are shared in pairs.
A pair of shared electrons between two atoms results in the formation of a covalent bond. The nitrogen atom forms 3 covalent
bonds or shares 3 pairs of electrons with the hydrogen atoms. By sharing electrons both the nitrogen atom and the hydrogen atoms will end up with a noble gas electron configuration. Hydrogen
only needs to gain 1 electron to fill its outer electron shell so each hydrogen atom will only form 1
covalent bond; this is outlined in the image below:

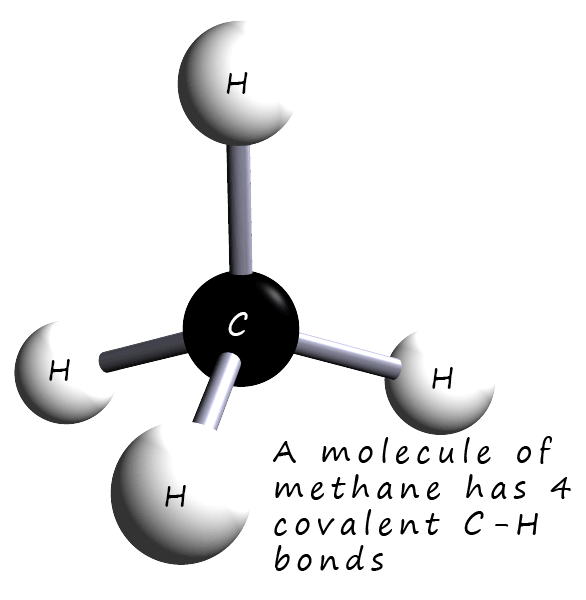
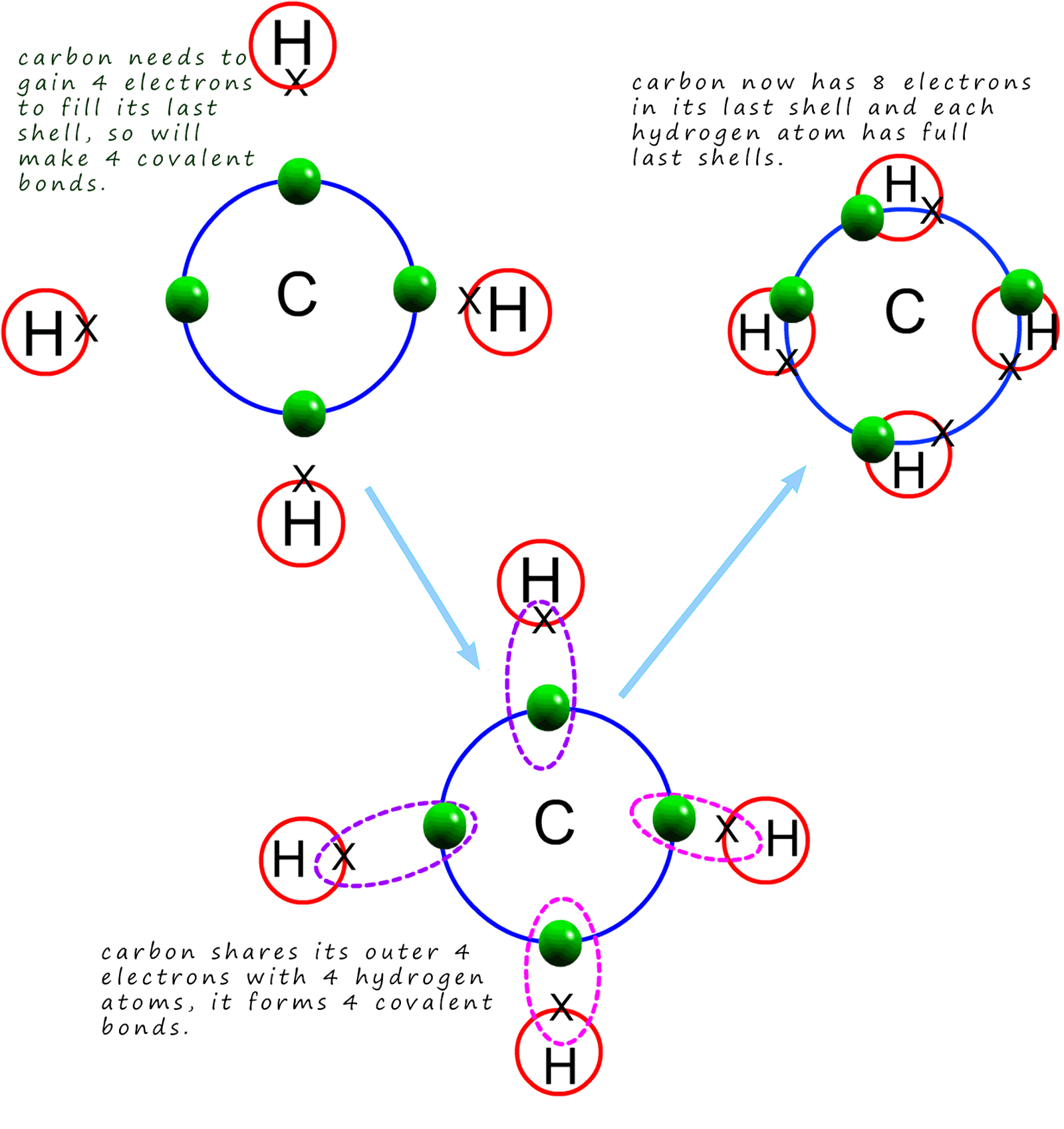
Methane is the gas we use to heat our homes and for cooking with; it is also the gas used in Bunsen burners in the science lab.
Its molecular formula is CH4 and it is a
covalent compound consisting of 1 atom of
carbon and 4 atoms of hydrogen. Carbon has an electron arrangement of 2,4 or an electronic configuration of 1s22s22p2.
Looking at the electron arrangement for carbon then if we apply the octet rule each carbon atom needs to gain 4
electrons to completely fill its outer shell so it will make 4 covalent bonds.
Each hydrogen atom only needs to gain 1 electron
so it will only make one covalent bond. In the dot and cross diagram below you can clearly
see that each carbon atom bonds with 4 hydrogen atoms. The final diagram below shows a dot and cross diagram for the methane
molecule, remember that a covalent bond involves the sharing of 2 electrons.
One electron comes from each of the atoms involved in
forming the covalent bond.
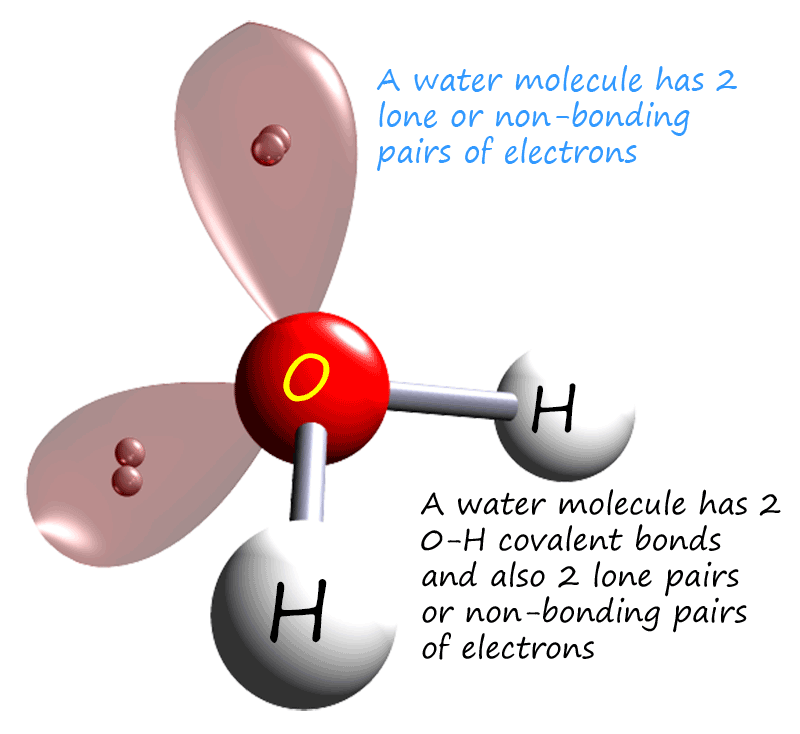
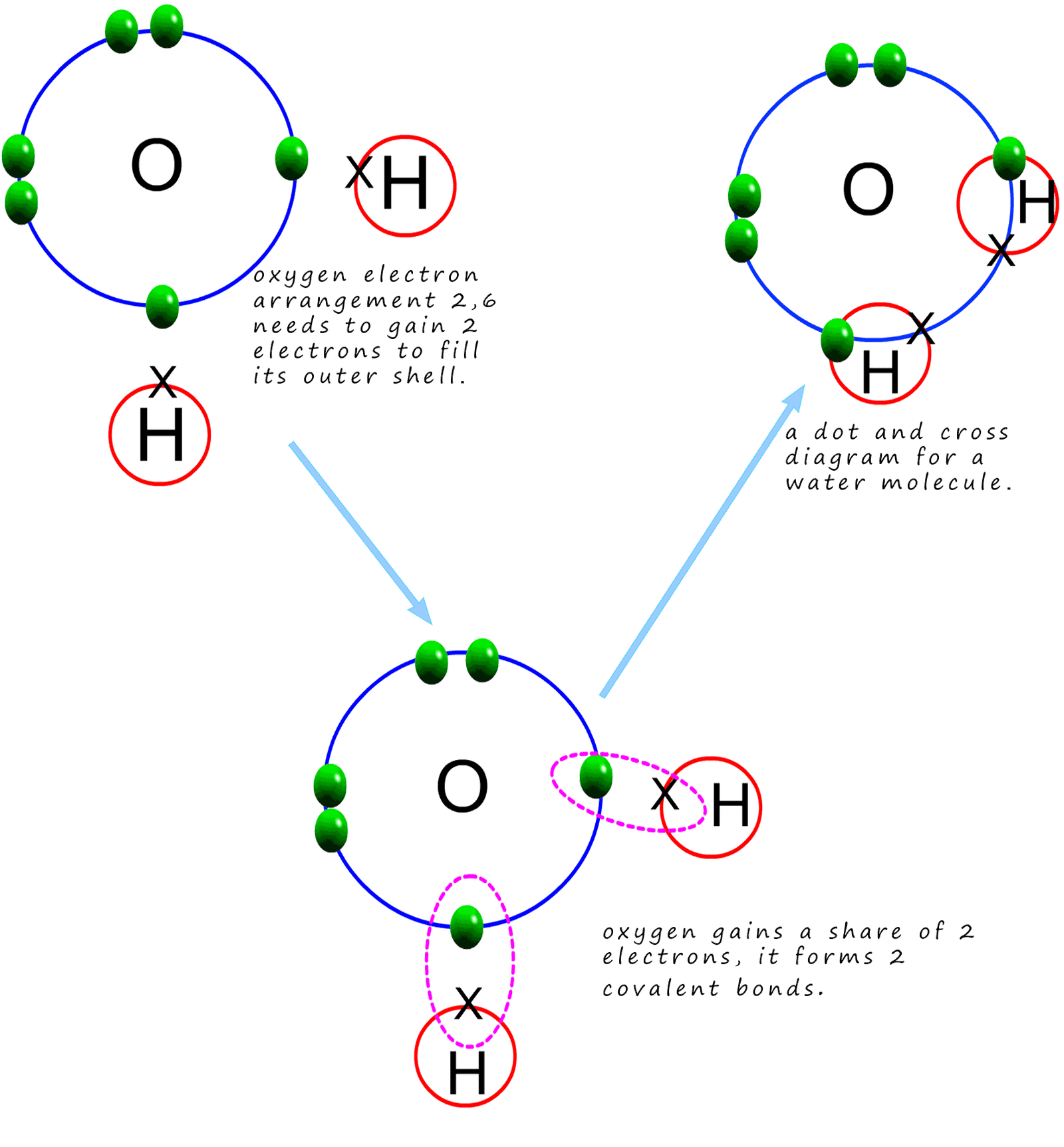
Oxygen is a group 6 element with an electron arrangement of 2,6 or an electronic configuration of 1s22s22p4. So if we apply the octet rule here the oxygen atom needs to gain 2 electrons to obtain a full outer electron shell and as before hydrogen only needs to gain one electron to fill its outer electron shell. To gain 2 electrons each oxygen atom will make 2 covalent bonds while each hydrogen atom only needs to gain 1 electron and so it will form only one covalent bond. Every atom in the water molecule ends up with a full last shell of electrons or a noble gas outer np6 electron arrangement. The oxygen atom also has 4 electrons in its last shell which are not used in bonding; so these electrons will form the 2 lone pairs as shown in the image below and opposite:
If atoms in a molecule share only 2 electrons then they will form single covalent bonds.
However if atoms have to
share more than 2 electrons in order to achieve full outer electron shells then double or even triple
covalent bonds
will be formed e.g. study the dot and cross diagrams below for hydrogen, oxygen and nitrogen molecules.
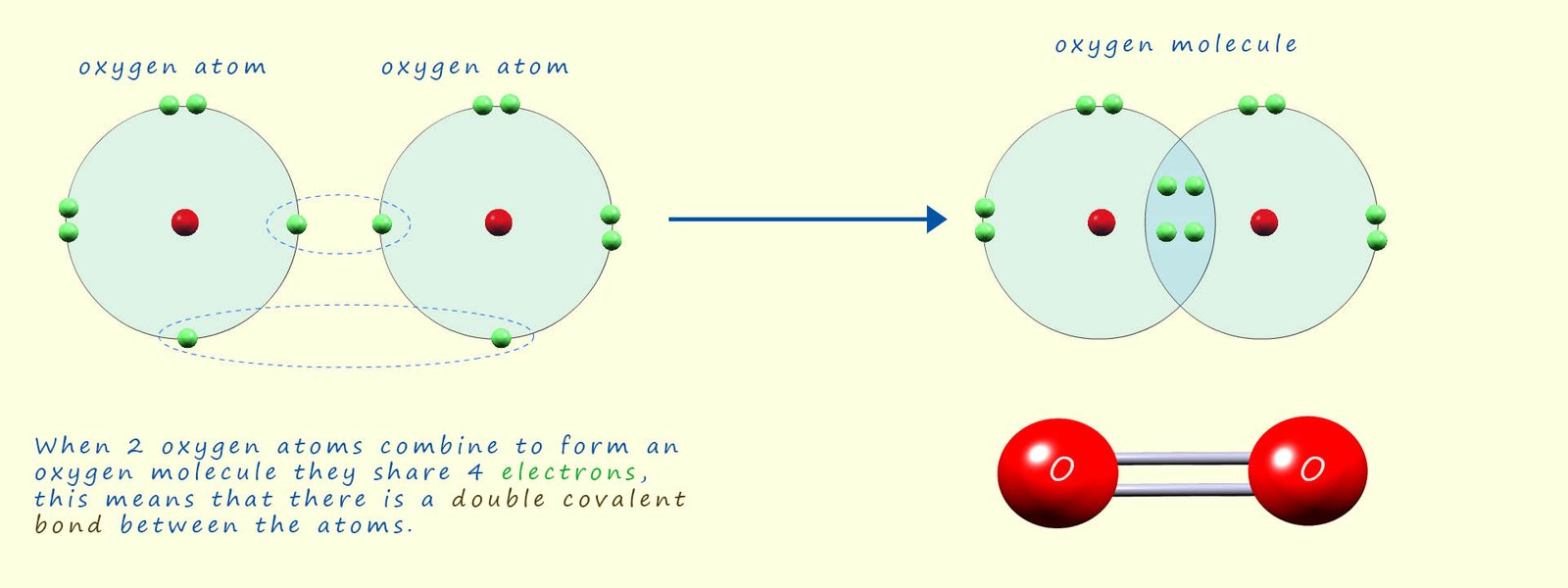
Oxygen atoms have 6 electrons in their outer electron shell so they will need to gain two electrons to achieve a stable octet or full outer electron shell; so when oxygen atoms join together to form an
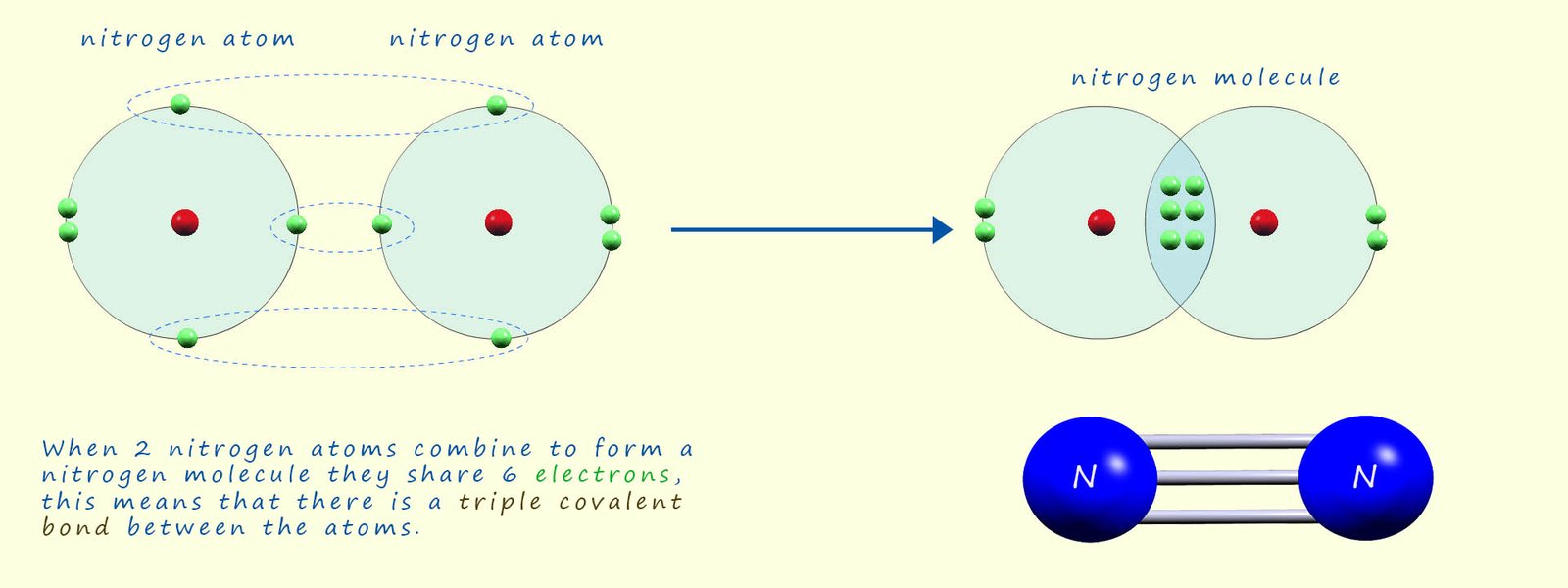
oxygen molecule these molecules will contain double covalent bonds between the atoms. Nitrogen atoms having only
5 electrons in their outer electron shell need to gain 3 more
electrons to completely fill their outer electron shell;
so when nitrogen atoms join there will be six electrons in the area of overlap between the two nitrogen atoms, this means
that there is a triple covalent bond between the two nitrogen atoms. The image below explains this in more detail:
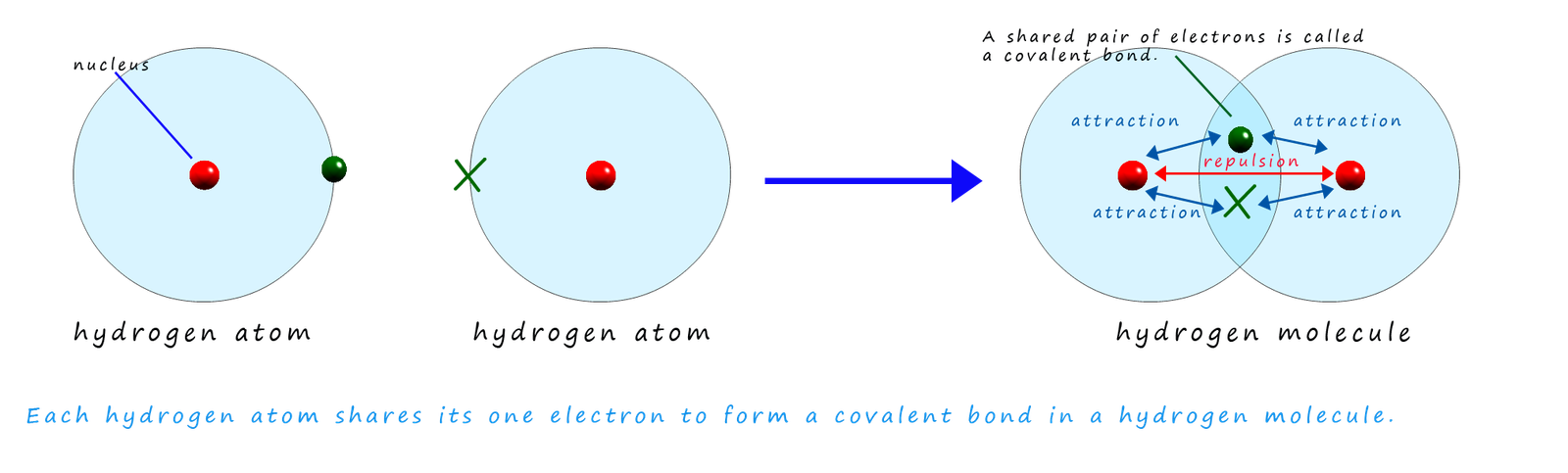
Hydrogen atoms contain only one electron and when two hydrogen atoms combine to form a hydrogen molecule they share 2 electrons and the molecule contains a single covalent bond as shown below:

Oxygen atoms have 6 electrons in their outer valency shell and so need to gain a further 2 electrons to achieve a stable octet of electrons. This means that when 2 oxygen atoms combine to form an oxygen molecule this molecule will contain a double covalent bond, that is the oxygen atoms share 4 electrons as outlined below:
Nitrogen being a group 5 element has 5 electrons in its outer electron shell. This means that it needs to gain 3 electrons to form a stable octet of electrons, so nitrogen atoms when they combine to form a nitrogen molecule will share 6 electrons and form a triple covalent bond. This is the main reason why nitrogen is such an unreactive gas; it takes a large amount of energy to break this triple covalent bond.
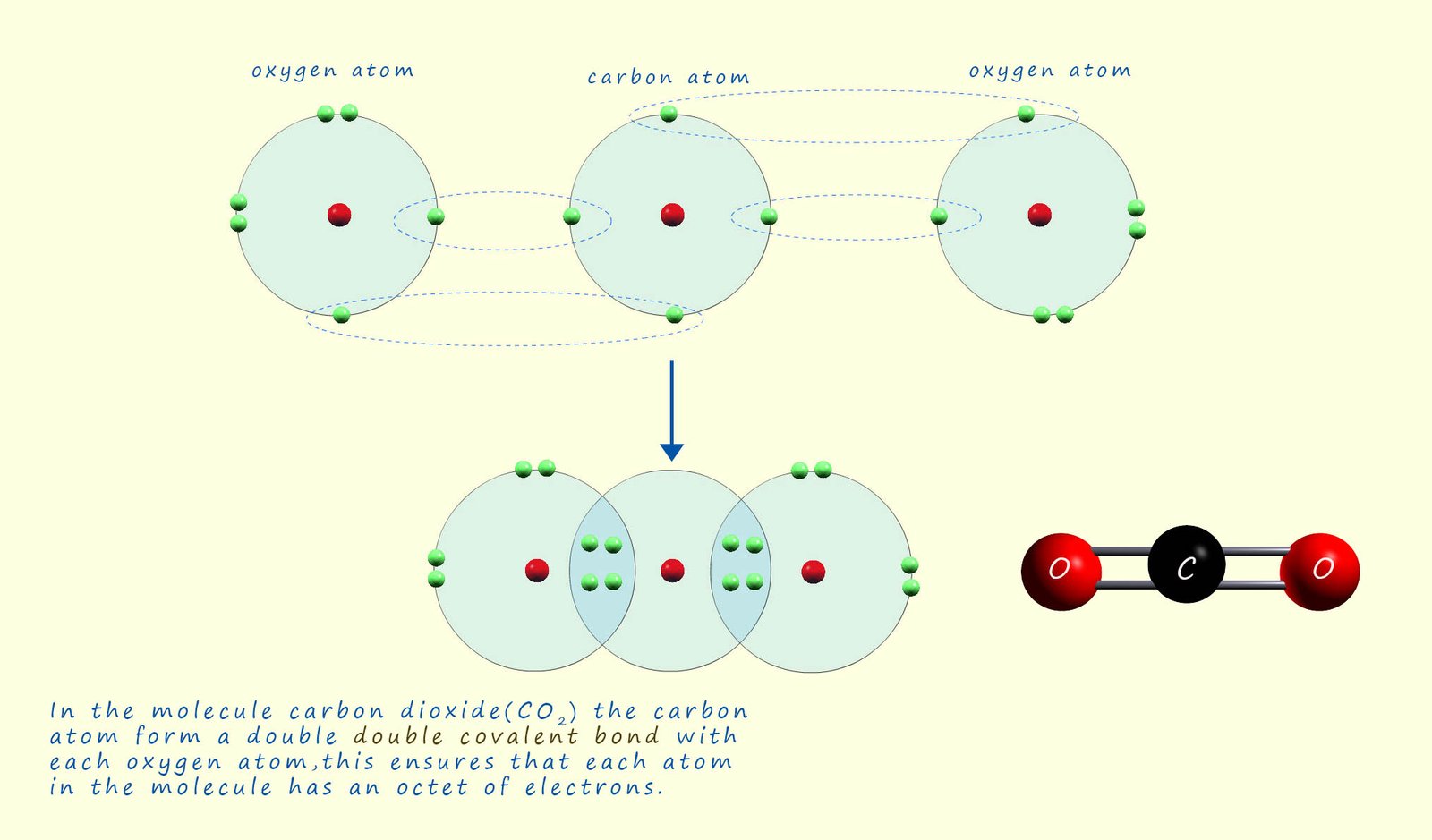
Carbon dioxide (CO2) is small covalent molecule that
contains a double
covalent bond between the atoms of carbon and
oxygen. Carbon has an electron
arrangement 2,4 and so it needs to gain a further 4 electrons
to complete its octet of electrons. Oxygen has an
electron
arrangement 2,6 so needs to gain 2 electrons to fill its outer electron shell.
In order for both the carbon and oxygen atoms to end up with
full last shells they share 4 electrons between them. This results
in a double C=O covalent bond forming. The diagram below
outlines how the carbon dioxide molecule forms: