
You are probably familiar with ionic bonds and covalent bonds from gcse chemistry. Before looking at polar covalent bonding it might be helpful to quickly re-cap ionic and covalent bonding.
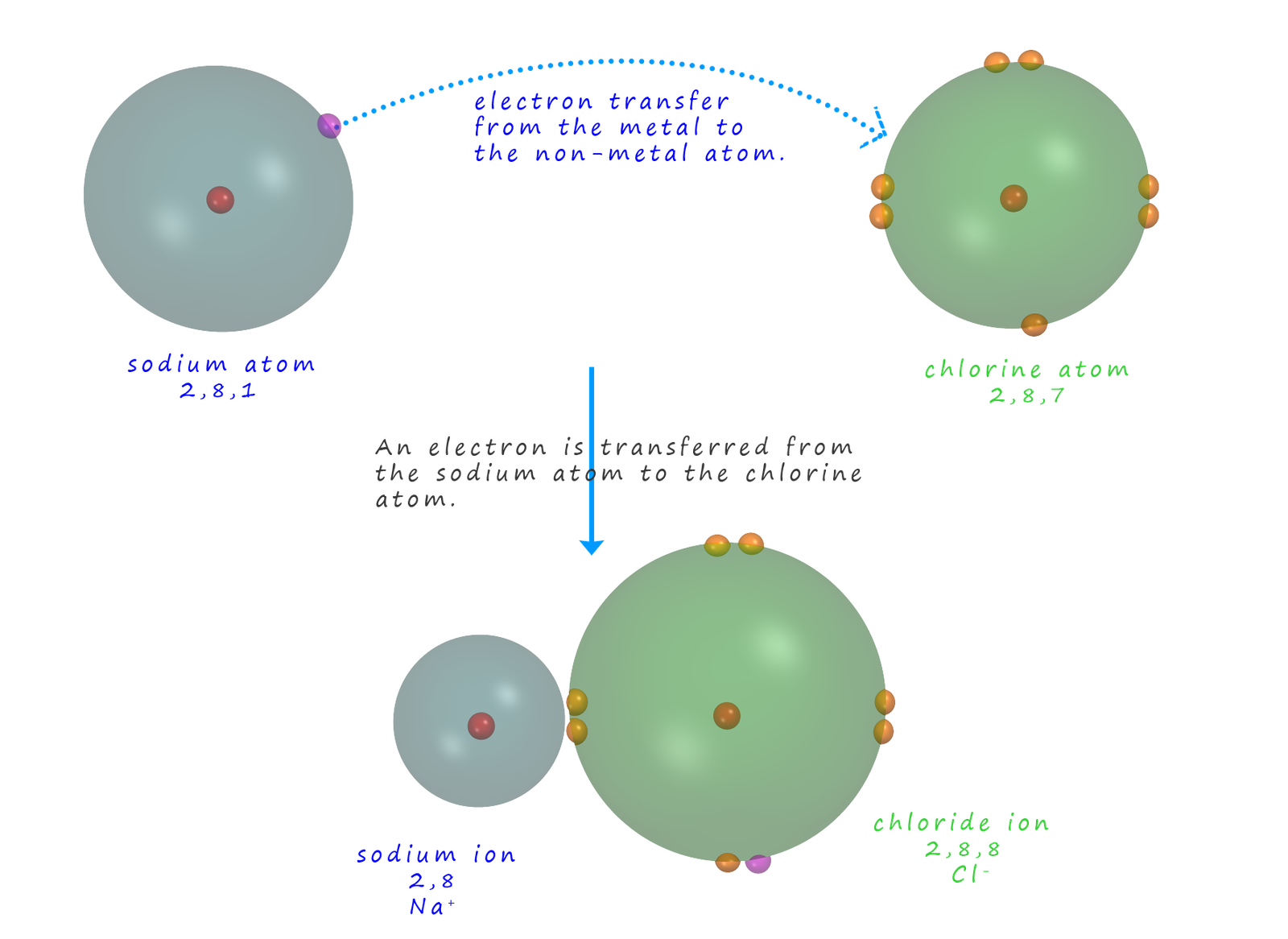
Ionic bonding involves the transfer of an electron from a metal to a non-metal atom, this results in the formation of ions with positive and negative charges. In the example shown below the metal sodium reacts with the non-metal chlorine to form the ionic compound sodium chloride. Here the metal sodium loses its outer shell 3s1 electron to chlorine. This means that the sodium atom forms a sodium ion with a charge of 1+. The non-metal chlorine gains an electron from the sodium and forms a chloride ion with an electron arrangement of 2,8,8 or an electron configuration of 1s22s22p62s23p6, this is outlined in the image below:
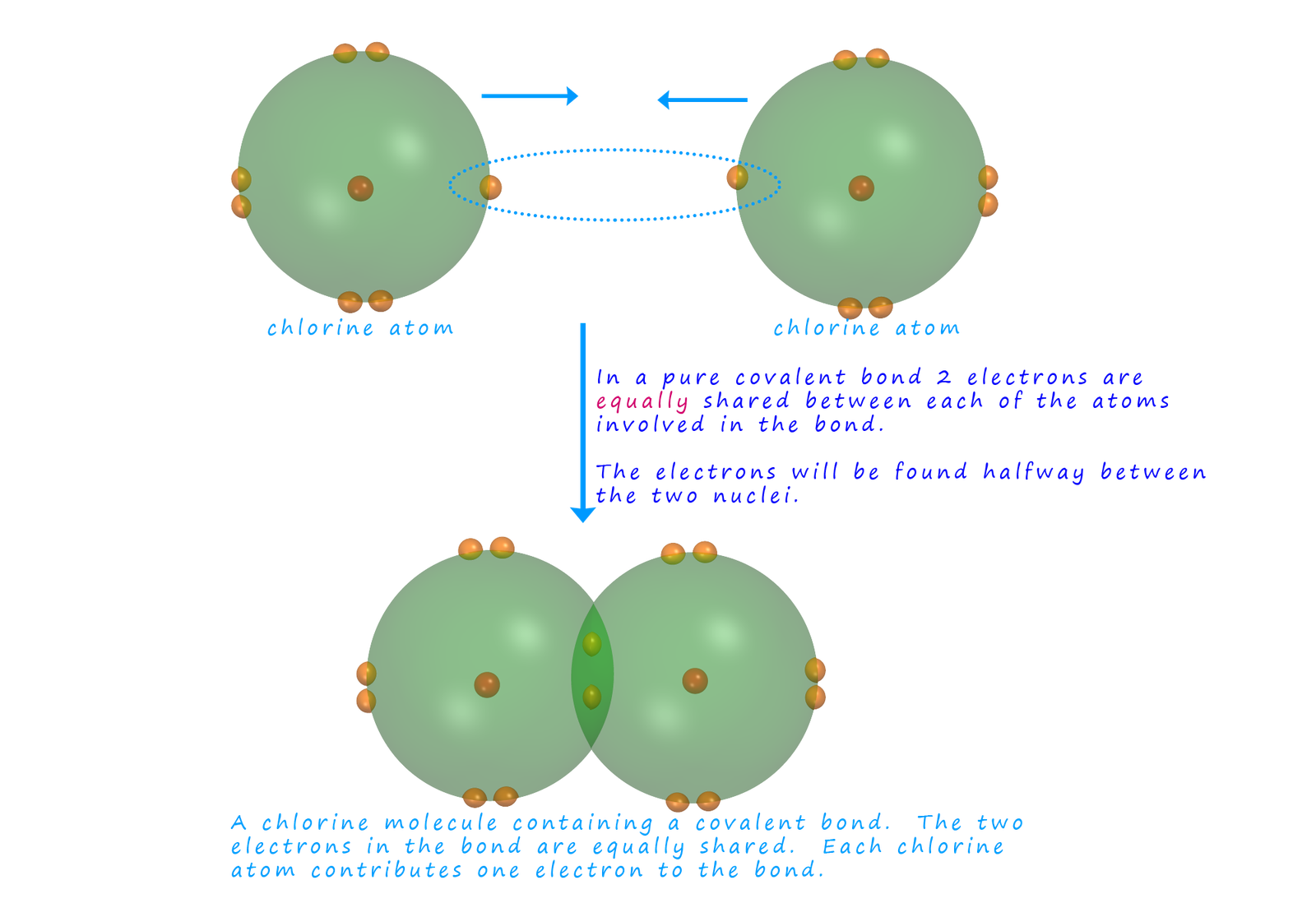
Most covalent compounds involve bonding between elements with different
electronegativities. This means that the electrons
in any bonds formed will NOT be shared EQUALLY.
In an ionic compound the ions formed
have positive and negative charges
because there is complete transfer of
an electron from the metal to the non-metal atom. In a
polar covalent bond the electron
is shared unequally between the
two atoms or we could simply say one atom in the bond has a much larger share of the
electrons. This means that
the atoms will have partial charges and not
full positive and negative charges.
The Greek symbol delta (lowercase)
δ is used to show the
resultant partial positive (δ+) and negative (δ-) charges that result from
the unequal sharing of the electrons in a polar
covalent bond.
A polar covalent bond or simply a polar bond can be thought as a sort of halfway house between a
covalent and an ionic bond; in that
the electrons in the bond are shared but not equally as is the case in a
covalent bond, this is why polar covalent bonds
contain atoms with partial charges and not full charges similar as found in an ionic compound. The charges on the atoms are only partial charges simply because the electrons are not completely transferred,
just shared unequally; this is outlined in the image below:
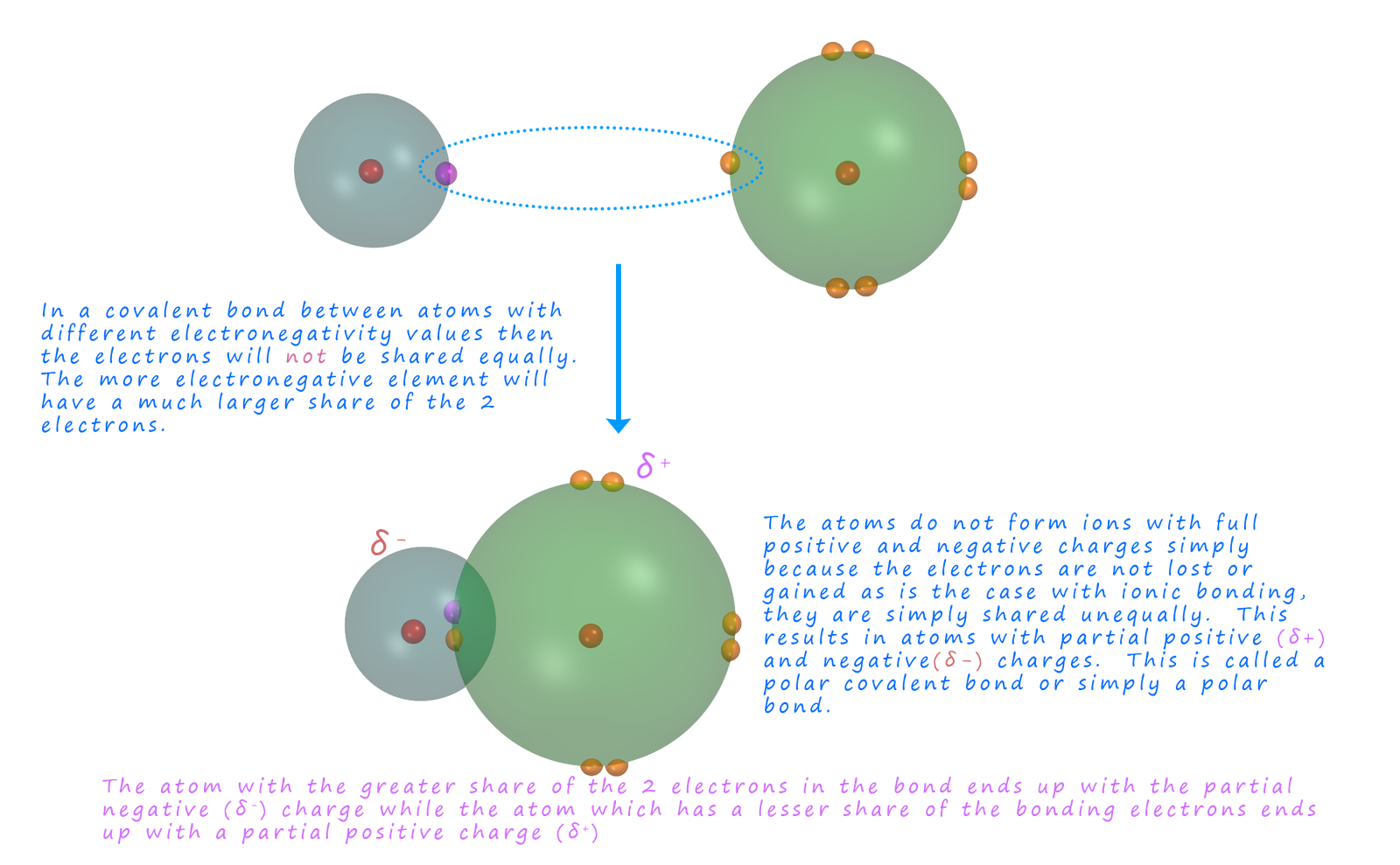
The larger the difference in the electronegativity values between the two atoms in the chemical bond the more polar (polar covalent) the bond will be. If the bond between two different atoms has a very large difference in electronegativity values of say 1.7-2.0 or more it will be ionic. If the differences in the electronegativity values are around 0.5 or less then the bond is likely to be covalent. Click here for more information on bond character and the degree of ionic or covalent nature in a chemical bond.
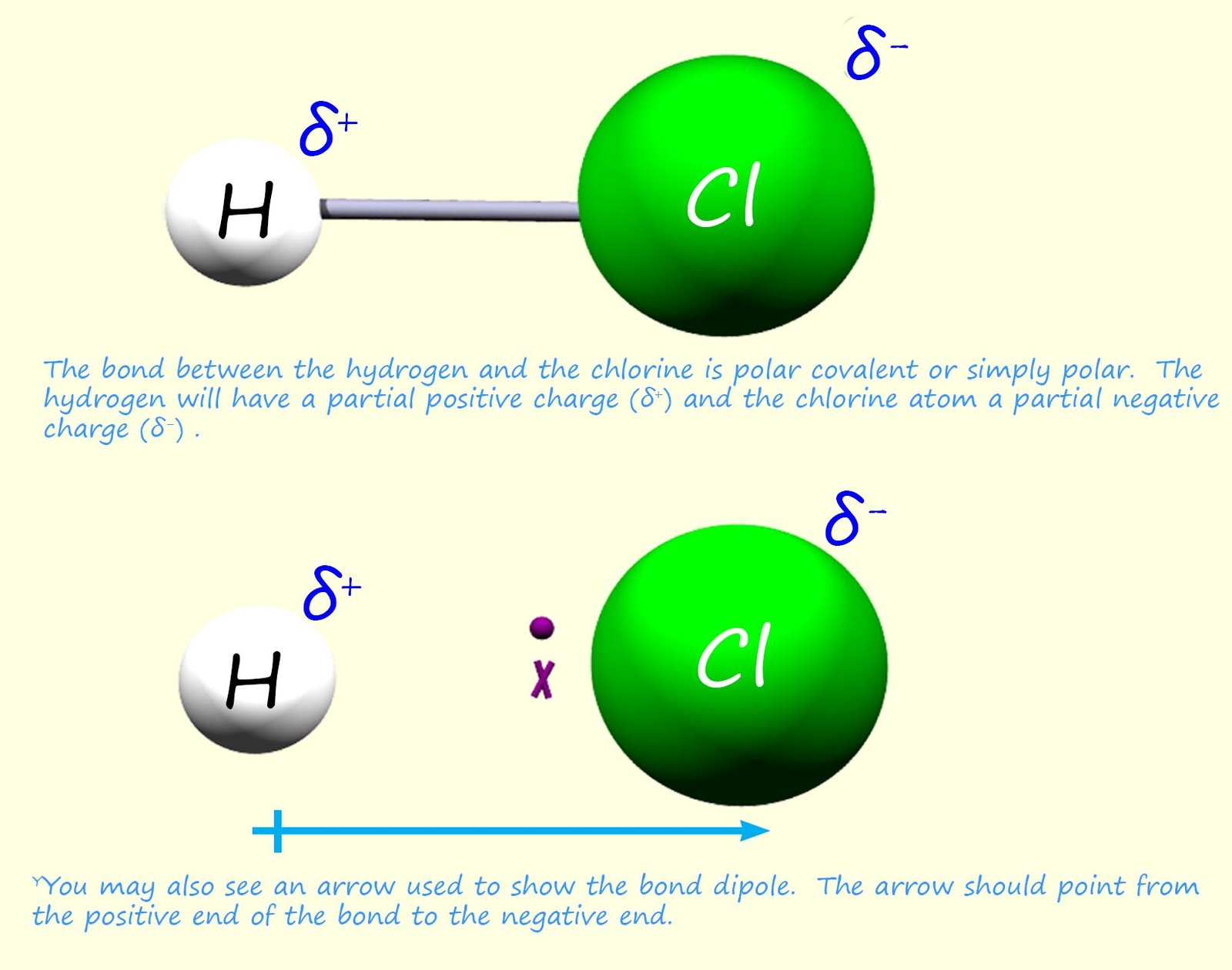
Consider a molecule of hydrogen chloride (H-Cl). Chlorine has an electronegativity value
of 3.0 and hydrogen has a value of 2.1; this
means that the bond is polarised
in such a way that the chlorine atom is electron rich (δ-)
and the hydrogen atom is
electron deficient (δ+). These two partially charged ends on the molecule are called poles; just like the opposite ends of a magnet are called poles. Now since the H-Cl bond has
2 charged ends it is described as having a bond dipole or
just a dipole (a dipole is simply
a small charge difference across a bond due to differences in electronegativities, which results in one end of the bond containing an atom with a partial
positive charge (δ+ ) and at the other end of the bond will be an atom with a partial negative charge
(δ-). The charges on both
end of the bond will be the same but different in sign, one negative and one positive.
Since H-Cl is a non-symmetrical molecule with polar
covalent bonds. We would also say that this is a polar molecule (that is one end of the molecule has a partial positive charge (δ+ )
and the other a partial negative charge (δ- ), this is shown in the image below:
Are all molecules which have polar covalent bond polar molecules? The answer to this question is NO. To decide if a molecule is polar you not only have to decide if it contains polar covalent bonds but you MUST also consider the shape of the molecule. Bond dipoles are vector quantities and have both a size and a direction. If the molecule has polar bonds but is highly symmetrical the chances are that the molecule will be non-polar even though it contains polar covalent bonds. As an example consider a molecule of carbon tetrachloride (CCl4) which is shown below:


You may also see the carbon tetrachloride molecule drawn out using arrows to represent the bond dipoles; as shown in the image opposite. The use of these arrows to indicate the size and direction of the bond dipoles is helpful
in deciding if the molecule overall will be polar with a dipole moment. The arrows representing the bond dipoles give
an indication of the size of the dipole present in any particular bond. I hope you can see that all the bond dipole arrows in this example will all effectively cancel each other out
due to the shape of this molecule. However if you study the examples below you will see that this is not always the case.
Consider each of the molecules shown below and then decide if they are polar or non-polar molecules. In each case you need to consider not only if the molecule has polar covalent bonds but also the shape of the particular molecule. Molecules which are highly symmetrical may have the centres of positive and negative charge directly on top of each other. If this is the case then the molecule will be non-polar despite having polar covalent bonds.
Carbon dioxide is a linear molecule, it is a symmetrical molecule which contains polar bonds; as shown in the image below; however is carbon dioxide a polar molecule?
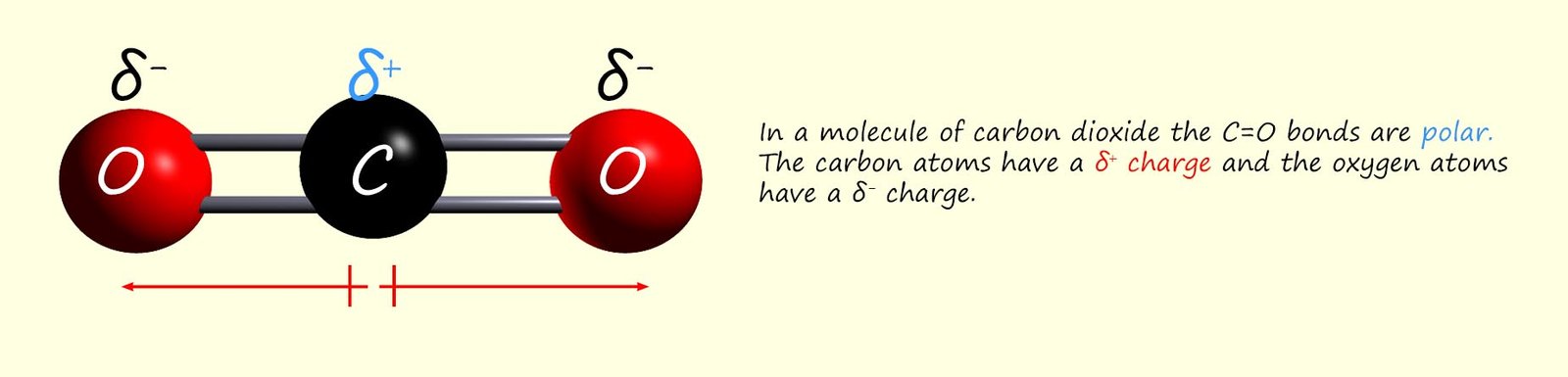
Despite having polar bonds carbon dioxide is not a polar molecule. The centre of positive charge on this molecule is directly centred on the carbon atom and the centre of negative charge is half way between the two oxygen atoms, that is directly over the carbon atom. This means that the centres of positive and negative charge overlap and this molecule, despite having polar bonds is a non-polar molecule.
 Being a symmetrical molecule means that for carbon dioxide the individual bond dipole cancel each other out leaving a non-polar molecule.
Being a symmetrical molecule means that for carbon dioxide the individual bond dipole cancel each other out leaving a non-polar molecule.
Boron trifluoride is another example of a non-polar molecule, despite the fact that it has polar bonds. The centres of positive and negative charge lie directly on top of the central boron atom. This means that due to its shape the individual bond dipoles for each of the B-F bonds add together and effectively cancel each other out.
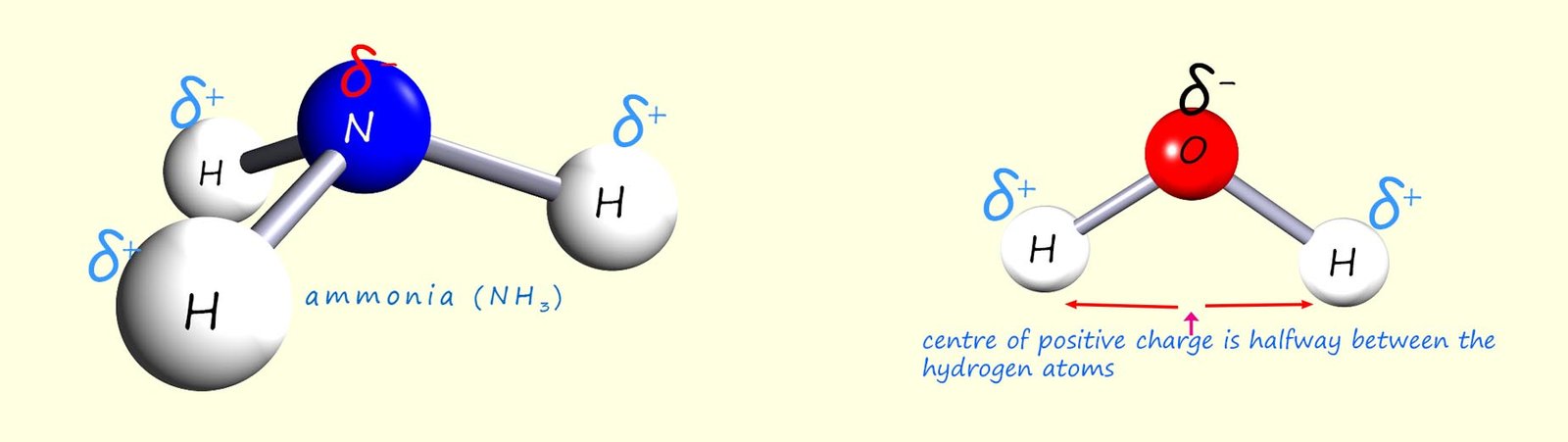
Finally consider two common molecules in chemistry, ammonia and water. Both these molecules have polar bonds as shown in the image below, but are the molecules themselves polar?