
Many atoms have non-bonding pairs of electrons. These non-bonding electrons pairs are often called lone pairs. Now these lone pairs of electrons can have a large affect on the chemical reactions, shape and physical properties of any molecules which contain atoms with these lone pairs of electrons. Many of the molecules you have already met in chemistry contain lone pairs; a few of the most common ones are shown below:

In a normal covalent bond between two atoms each atom contributes one electron to the bond and these electrons are then shared between the atoms in the covalent bond. As an example consider the single covalent bond formed between the chlorine atoms in a chlorine molecule, this is shown in the image below:

However there is another way in which a covalent bond can form, for example ammonia (NH3)
is a good base,
this means it will react with an acid (remember all acids contain H+ ions) by accepting a
hydrogen ion (H+) from the acid and neutralising it.
Now recall that a hydrogen ion (H+) is formed when a hydrogen atom loses it ONE electron. This means that it has no
electrons
available to form any new bonds. So how does the hydrogen ion react with an ammonia molecule to form a covalent bond with a nitrogen atom in an ammonia molecule if it
has no electrons?
The image below shows an ammonia molecule (NH3) and a hydrogen ion (H+) reacting together to form an ammonium ion (NH4+). Here a new covalent bond is formed between the nitrogen atom in the ammonia molecule and the hydrogen ion:
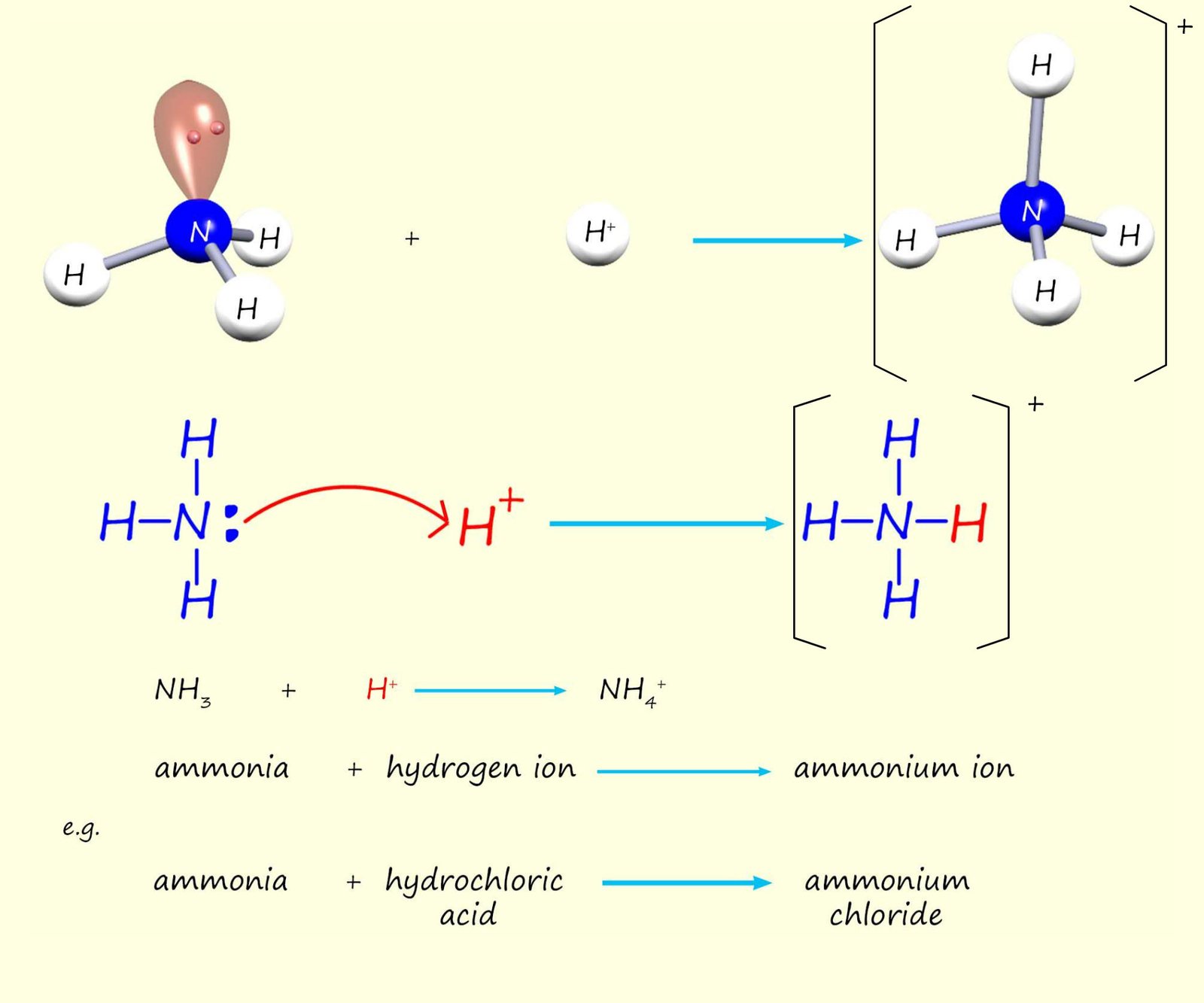
This reaction happens simply because the two electrons in the lone pair on the nitrogen atom supply BOTH the electrons in the new covalent bond that forms with the hydrogen ion from the acid. We can show this as:

 This type of bond where one atom supplies both the electrons in a covalent bond is called
dative covalent bond
or a coordinate bond, though the term coordinate bond is more commonly used when discussing the bonding involving metals, particularly in the context of coordination chemistry and complex ions. Once formed the dative covalent bond is no different from a
normal covalent bond.
However you should be able to identify that it was formed in a different manner from a normal covalent bond.
This type of bond where one atom supplies both the electrons in a covalent bond is called
dative covalent bond
or a coordinate bond, though the term coordinate bond is more commonly used when discussing the bonding involving metals, particularly in the context of coordination chemistry and complex ions. Once formed the dative covalent bond is no different from a
normal covalent bond.
However you should be able to identify that it was formed in a different manner from a normal covalent bond.
Dative covalent bonds are often shown in molecules with an arrow instead of the solid line used to represent a "normal bond" (see image opposite).
However you should be aware that the dative
covalent N-H bond in the ammonium ion has exactly the same in terms of bond strength and bond length as the other N-H bonds
in the ammonium ion. Once formed dative covalent bond are identical to normal
covalent bonds.
The hydrogen ion (H+) obviously has a positive charge and the ammonia molecule is neutral. So when the positively charged hydrogen
ion adds to the neutral ammonia molecule the new ammonium ion formed will have a positive charge.
Metals from groups 1 and 2 of the periodic table readily react with non-metals such as chlorine and oxygen to form ionic compounds which have a giant ionic lattice structures.However the transition metals are able to form both ionic compounds and also covalent compounds composed of molecules when they reat with non-metals, these molecules are often called complexes or complex ions and they contain coordinate bonds . For example the Cu2+ ion has empty d orbitals which are able to accept electrons from one of the lone pairs of electrons on a chloride ion. The copper ion (Cu2+) is able to form dative covalent bonds with 4 chloride ions. As mentioned earlier dative covalent bonds involving transition metals are often called coordinate bonds. This is outlined in the image below:

The Cu2+ ion has a 2+ charge and each chloride ion (Cl-) has a negative charge so the CuCl4 ion formed will have an overall charge of 2-.
Boron trifluoride (BF3) is an unusual molecule in that the boron atom at the heart of this molecule only has 6 electrons in its outer shell and not the 8 electrons you might expect based on the octet rule. These 6 electrons are from the 3 covalent bonds that the boron atom forms with the three fluorine atoms. Boron trifluoride is often described as an electron deficient molecule; meaning that it has space for another 2 electrons to complete its octet of electrons. However it has no free electrons available to form any covalent bonds so the only option to achieve 8 electrons in its outer shell is for the boron atom to form a dative covalent bond with a molecule containing a lone pair of electrons, for example boron trifluoride can form a dative covalent bond with an ammonia molecule; as shown in the image below:

Here the lone pair on the nitrogen atom in the ammonia molecule supplies both the electrons to form a dative covalent bond with the boron atom.
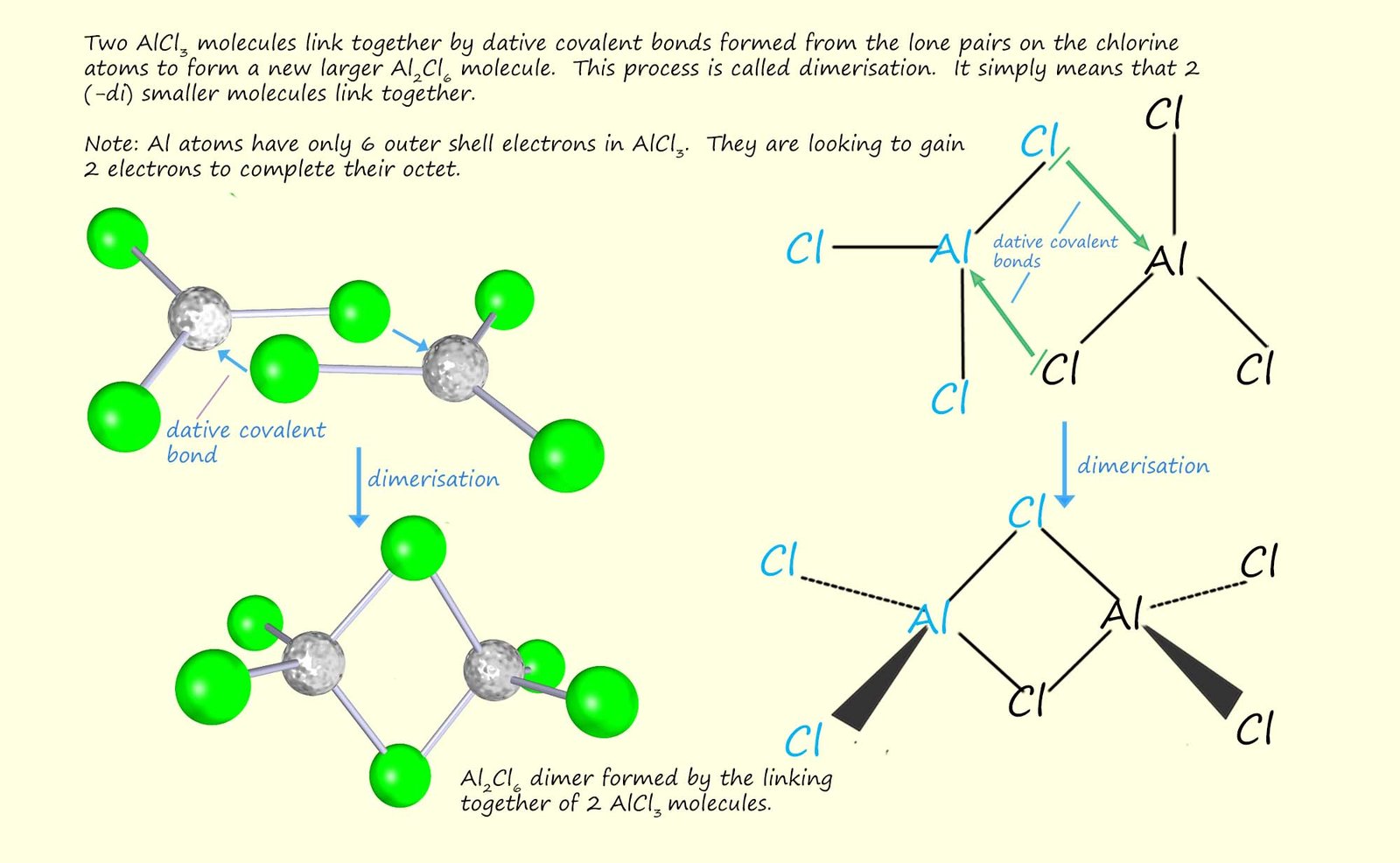
You might have expected aluminium chloride to be an ionic substance with a giant lattice structure, since it consists of a metal and a non-metal elements; however it is a covalent substance that consists of small AlCl3 molecules. The reason for this is to do with the polarising power of the small aluminium cation. In the gas phase aluminium chloride consists of small AlCl3 molecules as shown in the image below.

You should also note that the central aluminium atom makes only 3 covalent bonds to the chlorine atoms so this
means that it contains only 6 electrons in its outer valency shell, so like the boron atom in boron trifluoride the central aluminium atom is an electron deficient atom; in that it does have 8 electrons in its outer valency shell that might have been expected if it was obeying the octet rule. The aluminium atom will have empty orbitals that can accept
electrons that will
enable the aluminium atom to achieve a stable octet of electrons (8 electrons in its outer shell or a np6 noble gas electron
configuration).
The way in which aluminium achieves its octet of electrons is by forming dative covalent bonds with a chlorine atom on a neighbouring AlCl3 molecule. Each chlorine atom in AlCl3 has 3 lone pairs of electrons. The aluminium atom being electron deficient has no electrons available to form a covalent bond but it has empty orbitals that can accept electrons, so when the AlCl3 molecules in the gas state are cooled two AlCl3 molecules join to form a new larger molecule; Al2Cl6. This happens by the formation of 2 dative covalent bonds between the AlCl3 molecules as shown in the image below. This process is called dimerisation.
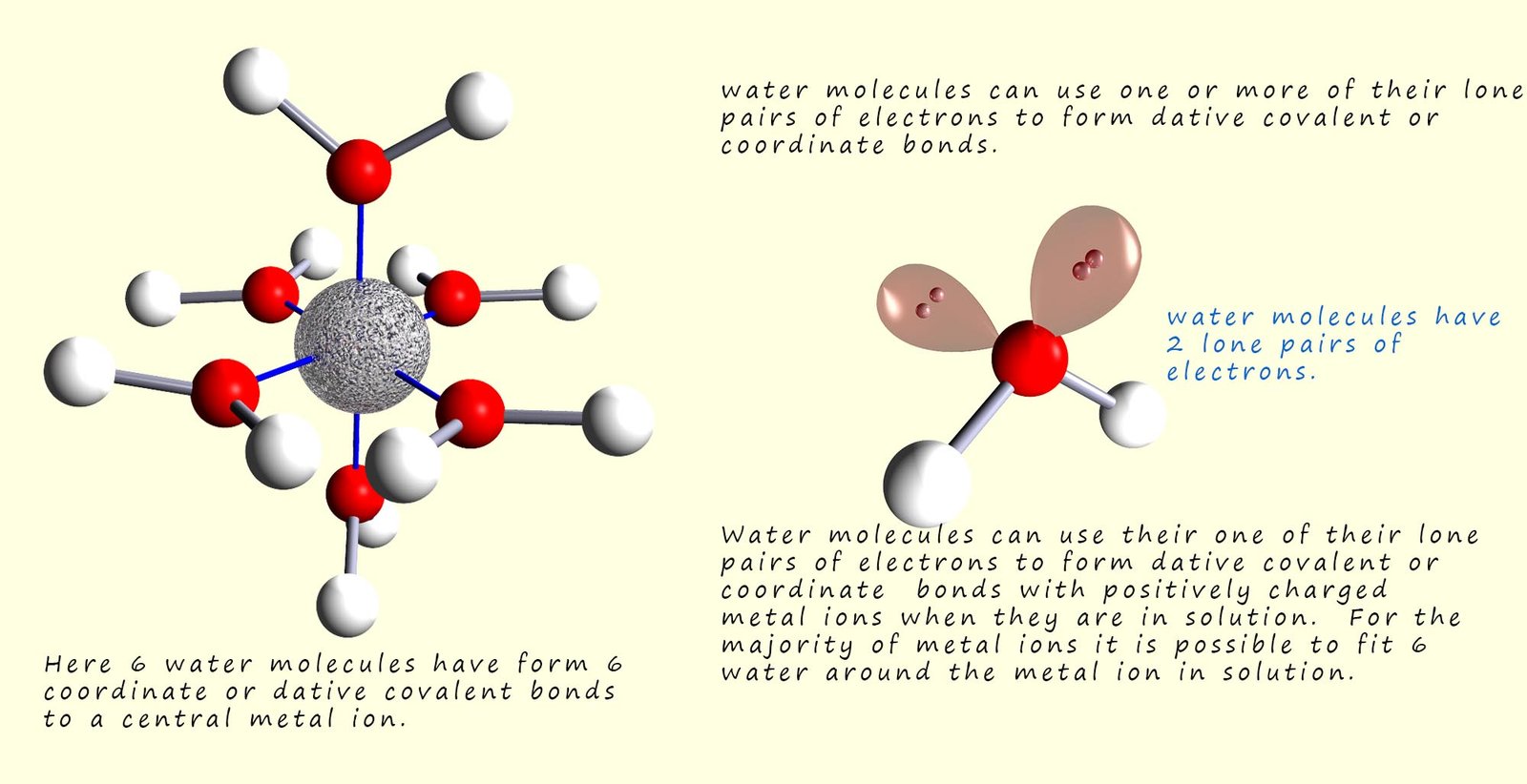
Water molecules can form coordinate or dative covalent bonds to metal ions which are in solution. The water molecules can use one of their lone pairs of electrons to form these coordinate bonds to the metal ions. The lone pair of electrons will bond to empty electron orbitals in the metal ion. Usually it is possible to fit 6 water molecules around a central metal ion to form a molecule or complex with the formula M(H2O)6. For example copper ions (Cu2+) can form a complex ion with the formula [Cu(H2O)6]2+ while aluminium ions in solution form a complex with the formula [Al(H2O)6]3+. This is outlined in the image below: