

The positively charged metal ions and the negatively charged non-metal ions produced when metals and non-metals react to form an ionic compound will be attracted to each other and pack as closely together as possible in a regular ordered way. Structures with regular arrangements of atoms or ions are often referred to as being crystalline. The electrostatic attraction of oppositely charged - that is positive and negatively charged ions is called an ionic bond. Ionic compounds consist of a giant structure made of these ions. These giant structures of ions are often referred to as a giant ionic lattice. The smaller the ions and the more highly charged they are means that they can pack together more closely and form a stronger ionic bond.
Sodium chloride is an ionic compound formed when the group 1 metal sodium reacts with the halogen chlorine. Sodium will lose its outer valency electron and form an ion with a +1 charge while chlorine; a group 7 halogen will gain 1 electron and form an ion with a -1 charge. If you look at grains of sodium chloride under the microscope you will see that they have a cubic shape. The image below shows this cubic structure for the sodium chloride lattice. The green spheres represent chloride ions (Cl-) and the blue spheres sodium ions (Na+).

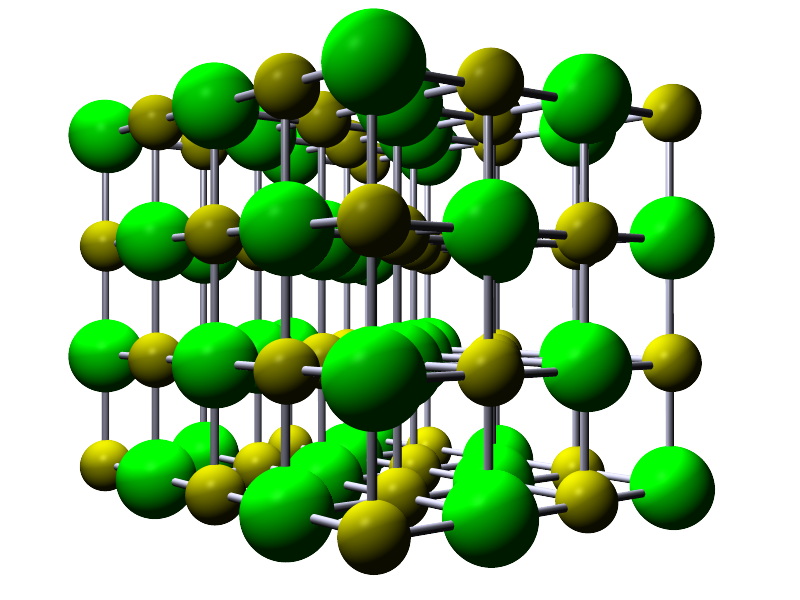 If you were to count the ions in the model shown opposite or in the image above you would notice that
there are equal numbers of sodium ions (Na+) and
chloride ions (Cl-). This is simply because when the ions pack together they do in
such a way that the charges always
cancel out. The +1 charge on the sodium ion is cancelled out by the -1 charge on the
chloride ion. So the ratio of
the ions present is 1:1. This simple ratio of ions enables you to calculate the empirical (simplest) formula for the ionic compound.
So in the case of sodium chloride it is NaCl. Its ionic formula is Na+Cl-.
If you were to count the ions in the model shown opposite or in the image above you would notice that
there are equal numbers of sodium ions (Na+) and
chloride ions (Cl-). This is simply because when the ions pack together they do in
such a way that the charges always
cancel out. The +1 charge on the sodium ion is cancelled out by the -1 charge on the
chloride ion. So the ratio of
the ions present is 1:1. This simple ratio of ions enables you to calculate the empirical (simplest) formula for the ionic compound.
So in the case of sodium chloride it is NaCl. Its ionic formula is Na+Cl-.
You will often see the lattice structure for ionic compounds shown in different ways. This is to try and help you
visualise what the giant lattice structure looks like. Opposite is shown the cubic lattice for sodium chloride (Cl- are shown in
green while the Na+ is a yellow/green colour), but this time there are bonds shown between the ions. In reality
there would be no gaps between the ions as they will pack together as closely as possible but it does allow you to view
the interior of the structure which is not possible from the first model, however both models allow you to visualise how the ions
are arranged in the lattice structure.
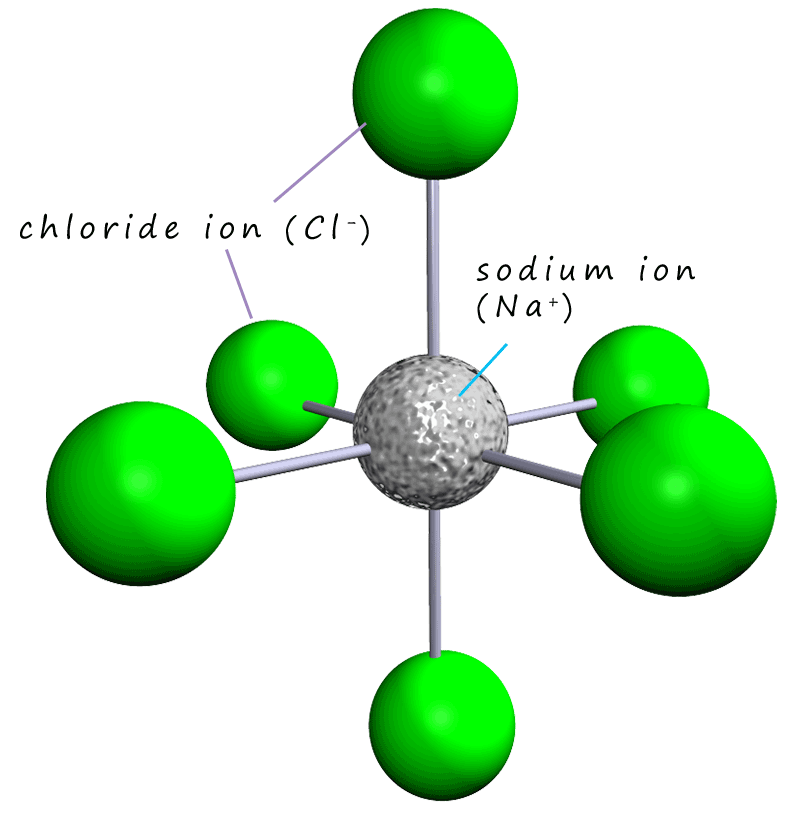 If
you pick out one sodium ion in the sodium chloride lattice you should see that it has six immediate neighbours. One
chloride above it and one below,
one to its left and one to its right and one in front and one behind, this is shown in the image opposite. The same is true if you
pick a chloride ion, it will also have 6 immediate neighbours; with each one being a sodium ion (Na+), since each sodium or chloride
ion has 6 immediate neighbours, the ions are described as 6 co-ordinate.
If
you pick out one sodium ion in the sodium chloride lattice you should see that it has six immediate neighbours. One
chloride above it and one below,
one to its left and one to its right and one in front and one behind, this is shown in the image opposite. The same is true if you
pick a chloride ion, it will also have 6 immediate neighbours; with each one being a sodium ion (Na+), since each sodium or chloride
ion has 6 immediate neighbours, the ions are described as 6 co-ordinate.
All ionic compounds have a giant lattice structure made up of positively charged and negatively charged ions. However not all ionic lattices have a face centred cubic
structure like sodium chloride. Remember that the ions will always try and pack together as closely as possible, but in a way that maximises the attraction between the oppositely charged ions and reduces the repulsion between ions of a similar charge. However
the different sizes of the ions in the compound will limit how closely they can pack together to maximise attractions and reduce repulsion.
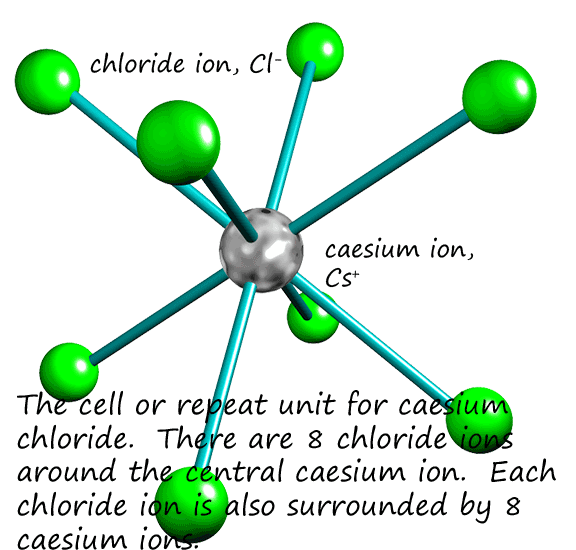 Caesium like sodium is an alkali metal in group 1 of the periodic table; however caesium being in period 6 is a much larger atom than sodium.
Caesium like sodium has one outer shell valency electron and so will form an ion with a 1+ charge (Cs+). This means that
caesium chloride will have the formula CsCl where the caesium and chloride ions are in the ratio of 1:1,
exactly the same as sodium chloride (NaCl). However caesium chloride has a different lattice structure from NaCl. The larger caesium
ion can fit 8 chloride ions around it; that is its coordination number is 8 compared to 6 with sodium chloride.
This is shown in the image opposite:
Caesium like sodium is an alkali metal in group 1 of the periodic table; however caesium being in period 6 is a much larger atom than sodium.
Caesium like sodium has one outer shell valency electron and so will form an ion with a 1+ charge (Cs+). This means that
caesium chloride will have the formula CsCl where the caesium and chloride ions are in the ratio of 1:1,
exactly the same as sodium chloride (NaCl). However caesium chloride has a different lattice structure from NaCl. The larger caesium
ion can fit 8 chloride ions around it; that is its coordination number is 8 compared to 6 with sodium chloride.
This is shown in the image opposite:
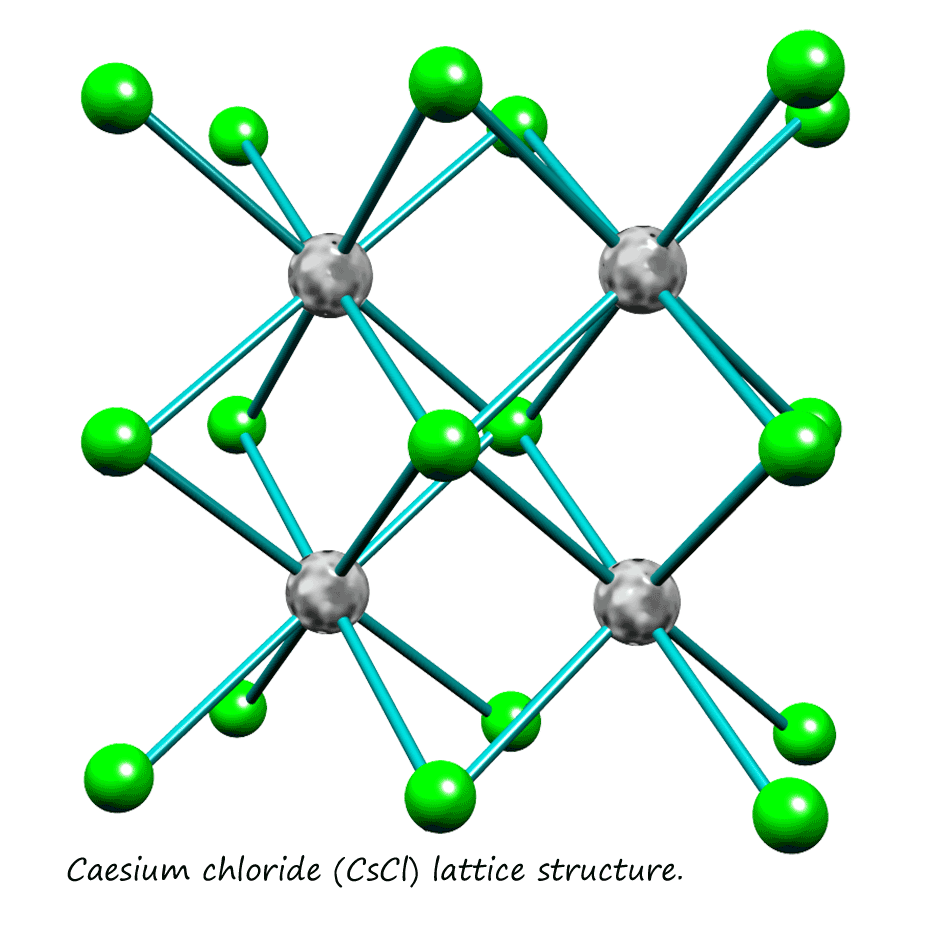
Note that in the cell shown for both the NaCl and the CsCl lattice the metal ion is shown at the centre with the chloride ions surrounding it. To visualise the coordination number
for the chloride ion simply swap the position of the metal ion and the
chloride ions around, put the chloride ion at the centre of the cell with the metal ions around it.
In caesium chloride you can visualise the caesium ions at the centre of a box with the chloride ions at the corner of this box. This type of structure is often referred to as body centred cubic (bcc). The coordination number for both the chloride and caesium ions is 8, this may be easier to visualise in the image below which shows a number of the CsCl cells that link to form part of the lattice structure for CsCl. The coordination number for each ion in the crystal must be the same since the ions are present in the ratio of 1:1.
The image below shows ball and stick models for the structure of the calcium fluoride lattice. Clearly it is not cubic like the sodium chloride lattice; this is simply because the ions are different in both size and charge. In this example the calcium ions (Ca2+) have a 2+ charge and are shown in red ions while and the blue ions are the fluoride ion (F-). Clearly the lattice structure for calcium fluoride is very different from that found in sodium chloride or caesium chloride; this is simply due to the way in which the ions pack together to ensure maximum attraction between the oppositely charged ions and to reduce the repulsion between ions with a similar charge.

When working out the empirical formula for an ionic compound it is only necessary to ensure that the charges on the ions cancel each other out e.g.
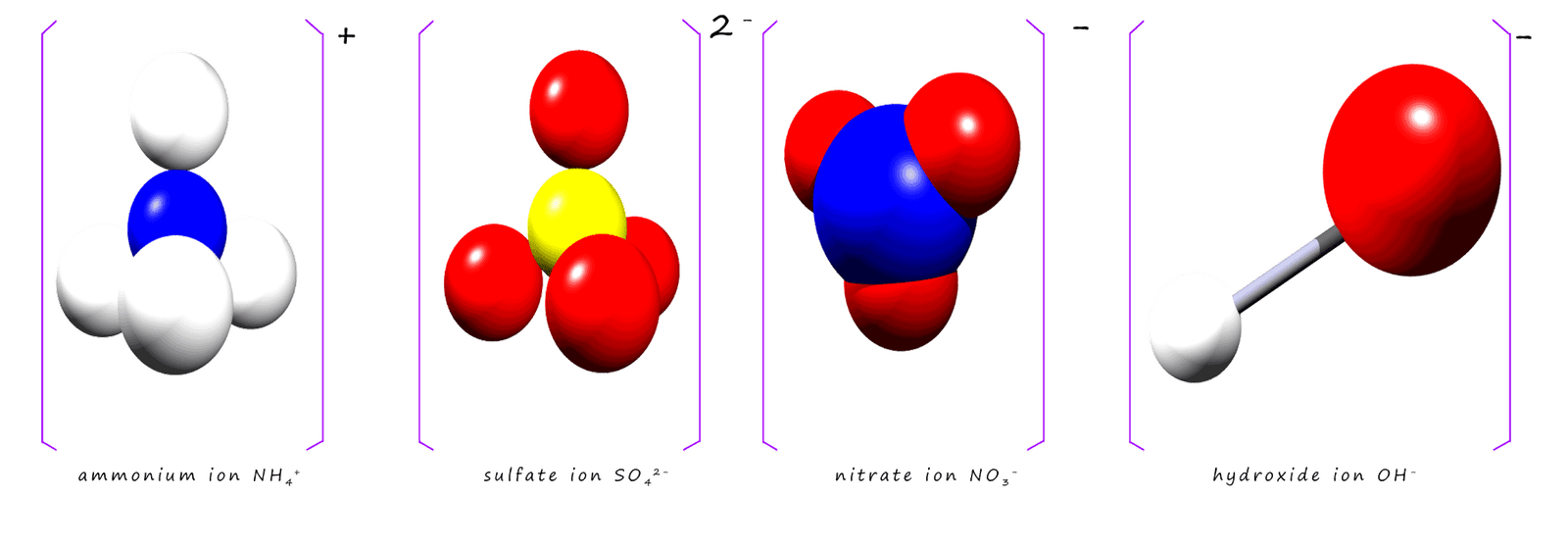 The formulae for the group ions you are likely to meet are given in the table below, it is perhaps worth making an effort to learn these formulae:
The formulae for the group ions you are likely to meet are given in the table below, it is perhaps worth making an effort to learn these formulae:
| Group ion | Elements present | Charge | Formula |
|---|---|---|---|
| hydroxide | hydrogen, oxygen | -1 | OH- |
| nitrate | nitrogen, oxygen | 1- | NO3- |
| sulfate | sulfur, oxygen | 2- | SO42- |
| carbonate | carbon, oxygen | 2- | CO32- |
| ammonium | nitrogen, hydrogen | 1+ | NH4+ |
| phosphate | phosphorus, oxygen | 3- | PO43- |
When working out the formula of compounds containing these ions we just use the same rules as before e.g.
| Ions present | sodium | sulfate | |
|---|---|---|---|
| Symbol | Na | SO42- | |
| Valency | 1 | 2 | |
| Swap over the valencies | 2 | 1 | |
| Ratio | 2 | 1 | |
| Formula | Na2SO4 | ||
| The ionic formula is simply the formula with the charges on the ions present. The charges on the ions are simply the number of electrons lost or gained when the element reacts. This is the same as its valency and for group ions it is probably easiest if you simply memorise the formula for these ions! | |||
| Ionic formula | Na2+SO42- | ||
| Ions present | calcium | nitrate | |
|---|---|---|---|
| Symbol | Ca | NO3- | |
| Valency | 2 | 1 | |
| Swap over the valencies | 1 | 2 | |
| Ratio | 1 | 2 | |
| Formula | Ca(NO3)2 | ||
| When writing out the formula for calcium nitrate the calcium ions and nitrate ions are in the ratio of 1:2. The brackets are required around the nitrate simply because if they are omitted then we have NO32, which reads as 1 nitrogen and 32 oxygen atoms, which is obviously not what we want. | |||
| Ionic formula | Ca(NO3)2- | ||