

The octet rule was used in gcse chemistry to help explain why elements reacted with each other and it also helped in working out the valency of the elements and the formula of any particular compound; it was assumed that the elements react in order to end up with full last electron shells or a noble gas electronic configuration. The octet rule assumed that elements end up with eight electrons or a ns2np6 electronic configuration in their outer electron energy level after they had reacted. However you will no doubt have noticed in your studies in A-level chemistry that there are quite a few molecules which do not appear to follow the octet rule. It would probably be fair to say that it's a rule and not a law. The octet rule tends to hold for most but not all of the first 20 elements in the periodic table; it would be fair to say that it is a rule and not a law and it should be used as a general guide. The octet rule tends to hold for most of the first 20 elements in the periodic table but there are many examples of elements which do not follow the octet rule.
The period 2 elements beryllium and boron can form compounds with fewer than eight electrons around the central atom. Boron has an electronic configuration of 1s22s22p1 or simply an electron arrangement of 2,3 and based on this electron arrangement we might predict using the octet rule that boron would lose 3 electrons or gain 5 electrons in order to complete its octet of outer electrons. However it does neither but instead forms three covalent bonds to form molecules with only 6 electrons in its valency shell. As an example consider the molecule boron trifluoride (BF3), a dot and cross diagram is shown below for this molecule. It is clear that the central boron atom has only 6 electrons in its valence shell. Aluminium another group III element also forms these trigonal planar molecules with only 6 electrons around the central atom. This would suggest that the octet rule does not apply to some group 3 elements. However these electron deficient molecules have empty electron sub-shells and are able to form dative covalent bonds which enable them to achieve an octet of electrons.
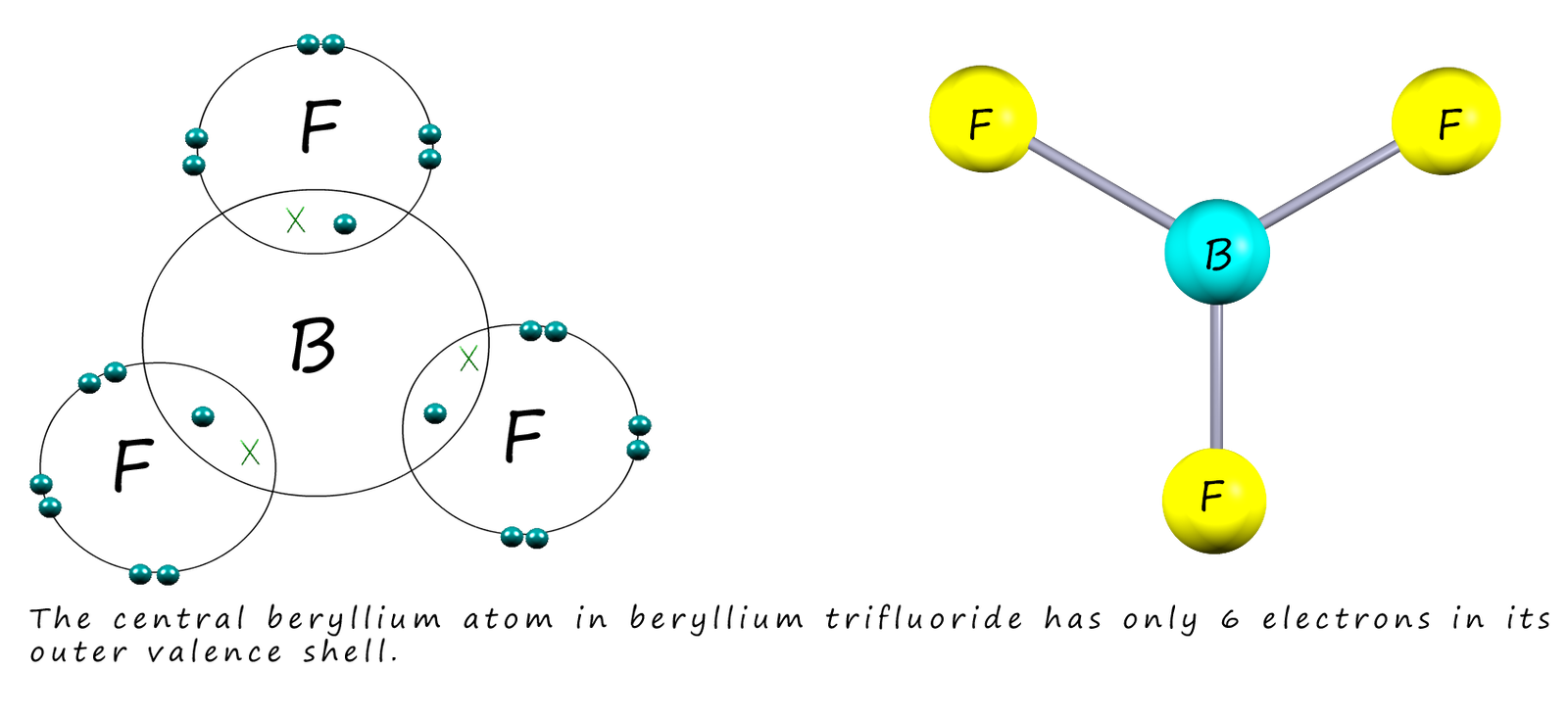
The group 2 element beryllium has an electronic configuration 1s22s2 and like boron also forms compounds which are electron deficient around the central atom. In beryllium chloride for example the central beryllium atom has only four electrons in its outer electron shell, this is shown in the image below:

There are many examples of molecules where the central atom has more than eight
electrons in its outer valency shell. These molecules
or ions with more than eight electrons around the central atom are often referred to as being hypervalent.
These hypervalent elements are found mainly in period 3 and above in groups 3, 4, 5, 6, 7 of the periodic table
though the noble gases krypton and xenon in group 0 can also form hypervalent compounds.
As an example consider the following:
 Phosphorus is an element in group 5 and period 3
of the periodic table that can form hypervalent molecules e.g.
Phosphorus reacts with chlorine to form 2 chlorides: phosphorus
trichloride (PCl3) and phosphorus pentachloride (PCl5).
Phosphorus is an element in group 5 and period 3
of the periodic table that can form hypervalent molecules e.g.
Phosphorus reacts with chlorine to form 2 chlorides: phosphorus
trichloride (PCl3) and phosphorus pentachloride (PCl5).
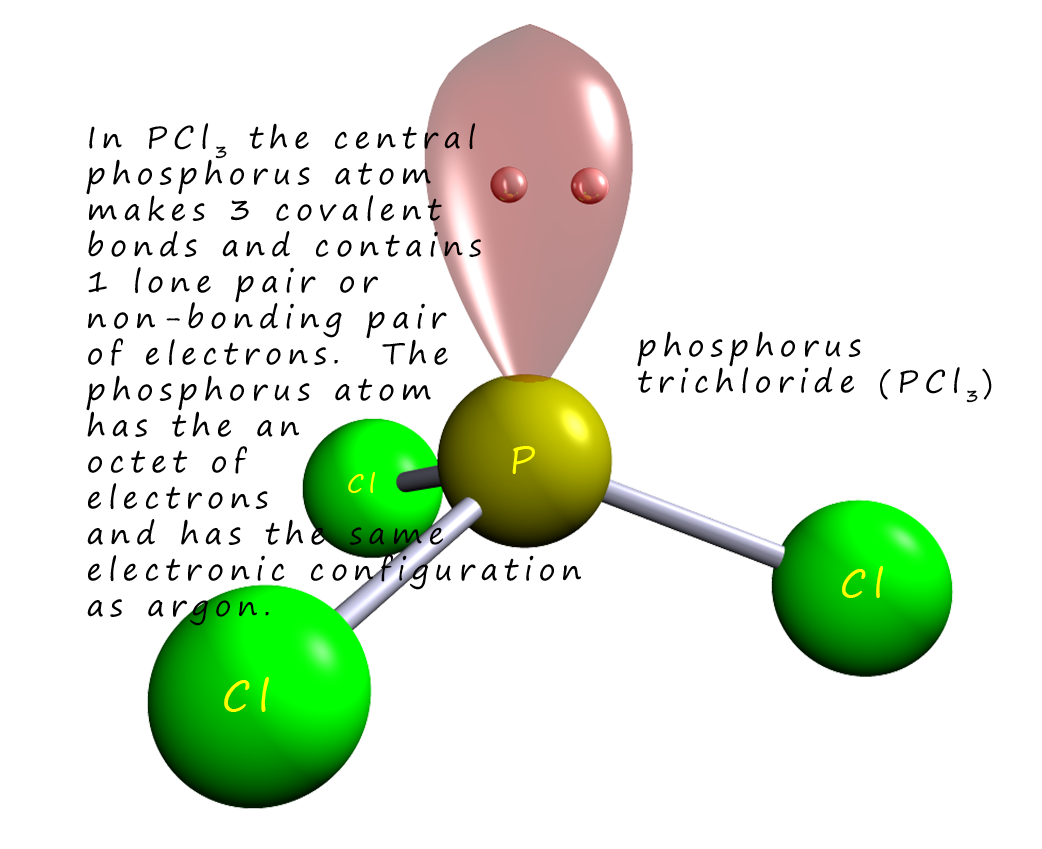
Now Phosphorus has an electronic configuration of
[Ne]3s23p3 indicating that it has 5 electrons in its valence shell. It can form three
covalent bonds
with chlorine and so gain a share of 3 additional electrons to form PCl3 which will give the central
phosphorus atom an octet of electrons and the same electronic configuration as the
noble gas argon.
However the 3d electron sub-shell in phosphorus is very close in energy to the 3s and 3p sub-shells and one
electron can be promoted from the 3s sub-shell to the empty 3d sub-shell. This will give phosphorus the new electronic
configuration:
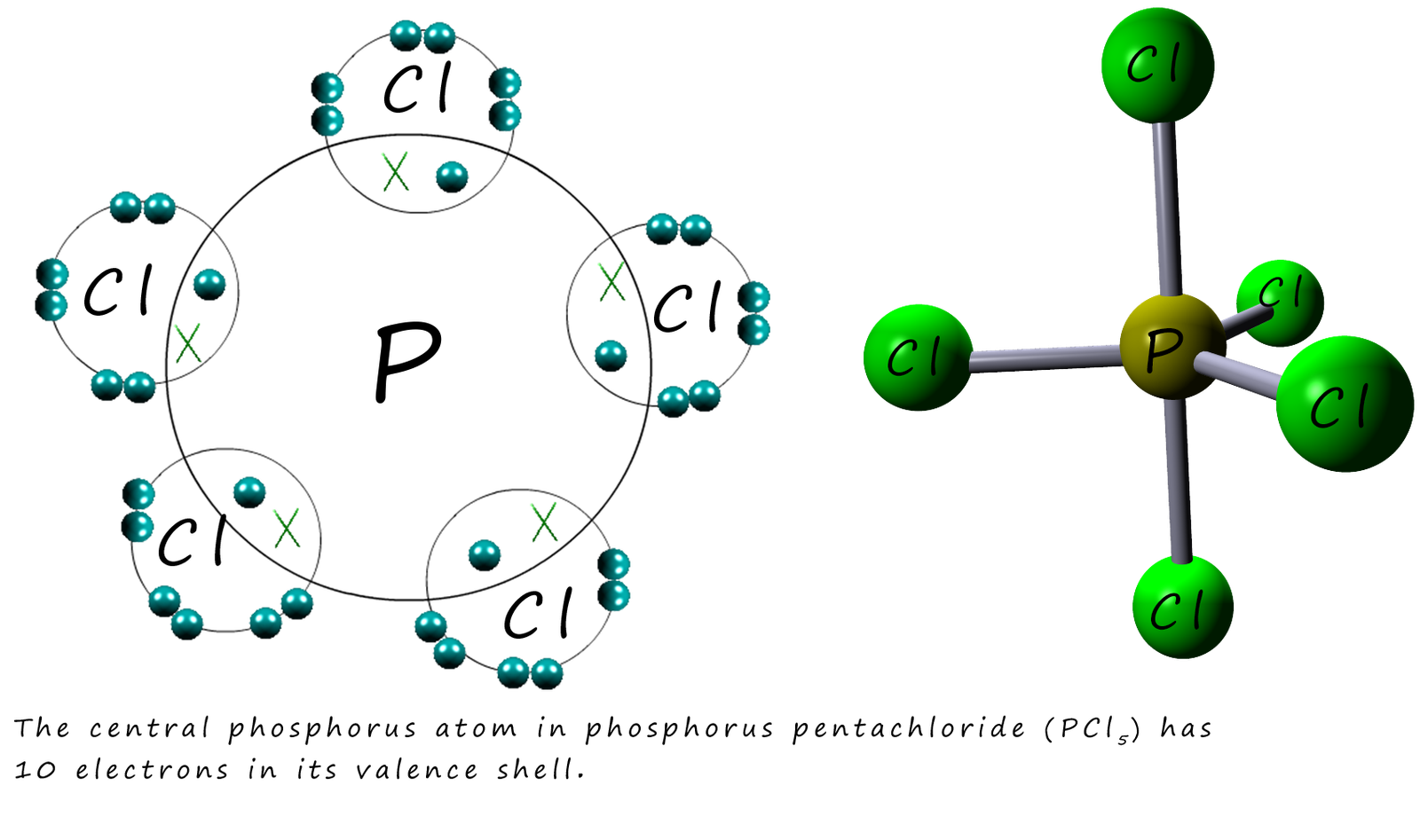
This new electron configuration now gives phosphorus 5 unpaired electrons in its valency shell and allows it to form the hypervalent chloride PCl5 where there are 10 electrons around the central phosphorus atom as shown below:
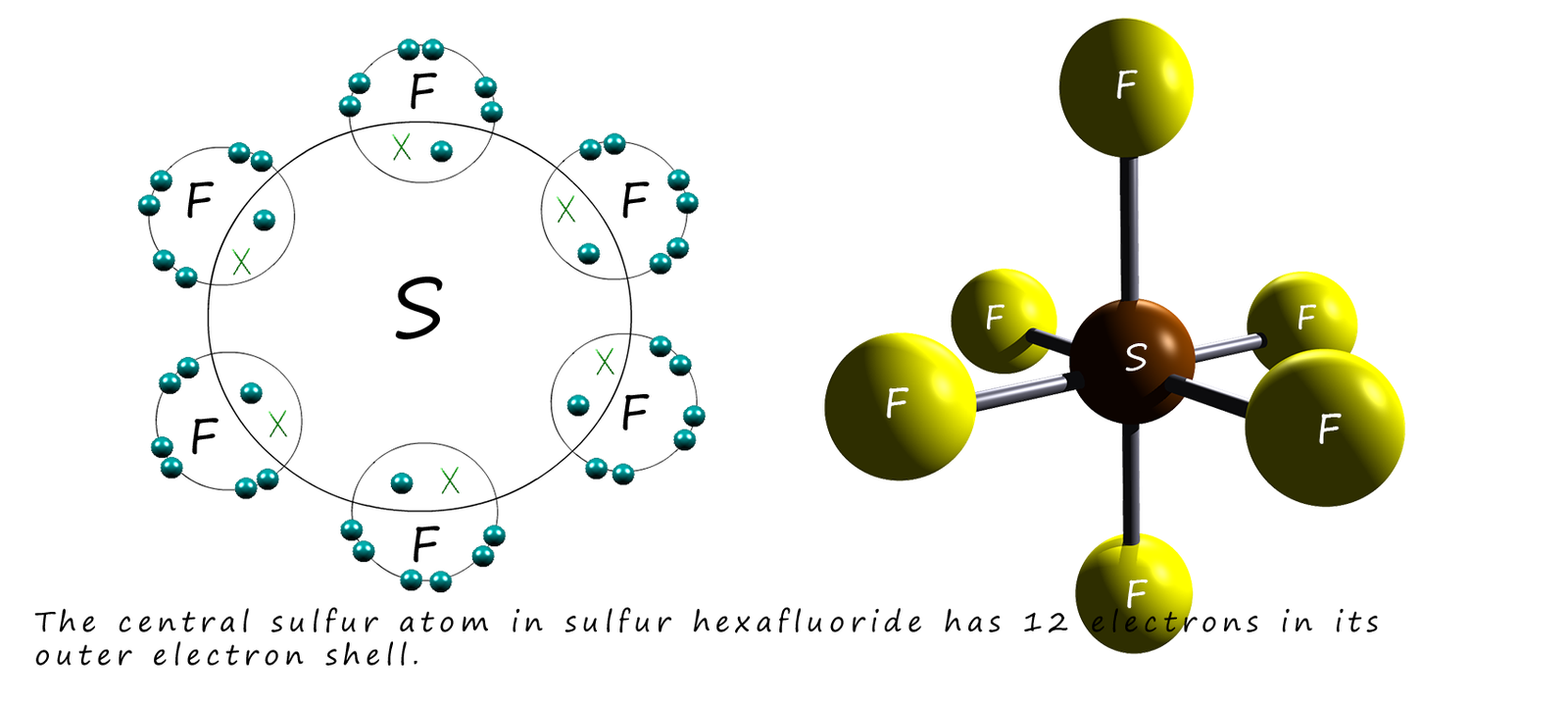
Sulfur has an electronic configuration of [Ne]3s23p4 indicating that it has 6 electrons in its valence shell. It can form 2 covalent bonds with another non-metal element and so gain a share of 2 additional electrons to form a compound which will give the central sulfur atom the same electronic configuration as the noble gas argon. Like phosphorus above the 3d electron sub-shell is very close in energy to the 3s and 3p sub-shells and so sulfur can promote 1 electron from the 3s sub-shell and also one electron from the 3p sub-shell into the empty 3d sub-shell. This will give the sulfur atom the new electron configuration of:
This new electron arrangement gives the sulfur atom 6 electrons in its valency shell and allows it to 6 covalent bonds to form hypervalent molecules such as sulfur hexafluoride (SF6) which has 12 electrons in its valency shell as shown below:
The two examples given above are just 2 of the many hypervalent molecules that exist where the central atom in a molecule is a non-metal element in period 3 and above. It is worth mentioning that in the two examples given above; PCl5 and SF6 that the corresponding molecules from the period above; namely NCl5 and OF4 do not exist and cannot be made. The main reason for this is due to the sizes of the central atoms. The period 3 elements P and S are large enough to fit 5 or 6 smaller F or Cl atoms around them whereas the smaller period 2 elements nitrogen and oxygen atoms simply cannot fit as many atoms around them. We should also consider the availability of the d sub-shell for use in bond formation. In period 2 elements such as N and O only the 2s and 2p sub-shells are available for bonding because the 3d sub-shell is much higher in energy.
Most of the molecules you will meet have an even number of electrons in their outer valency shell however there is a rather curious group of molecules which contain an odd number of electrons in their valency shell, this obviously means that they cannot have an octet of electrons in their last shell as you cannot pair an odd number of electrons!
The following two examples show simple molecules with an odd number of electrons in their valency shell:
Transition metals are elements found in the d-block of the periodic table, and they do not follow the octet rule in the same way as main group elements. This is because they have access to d orbitals in addition to the s and p orbitals. As a result, transition metals can have more than eight electrons in their valence shell and they often form compounds with variable oxidation states which again do not follow the octet rule.
 The unique behaviour of the transition metals arises from their electron configurations. For example, the electron configuration of the transition metal iron (Fe) is [Ar]4s23d6. Transition metals can lose different numbers of electrons to form cations with various charges, and this flexibility in losing electrons is the key to understanding their chemical behaviour.
The unique behaviour of the transition metals arises from their electron configurations. For example, the electron configuration of the transition metal iron (Fe) is [Ar]4s23d6. Transition metals can lose different numbers of electrons to form cations with various charges, and this flexibility in losing electrons is the key to understanding their chemical behaviour.
The oxidation states of iron:
The oxidation states of copper:
From the examples provided above we can see that the chemistry of the transition metals often do not follow the octet rule due to their ability to utilise d orbitals and form stable ions with various oxidation states. The chemistry of the transition metals is dominated by the availability and filling of the d-orbitals, leading to some unique and interesting chemistry that go beyond the simple octet rule applied to the main group elements.