


As the difference between the electronegativities values for the two atoms in a covalent bond increase the bond will become more polar. As the bond polarity increases the covalent bond becomes more ionic like in character. Eventually as the difference in electronegativity values rises from the two atoms forming the bond rise to around 1.7-2 the bond is more ionic than covalent and we consider it to an ionic bond with the usual properties we expect of an ionic compound, that is:

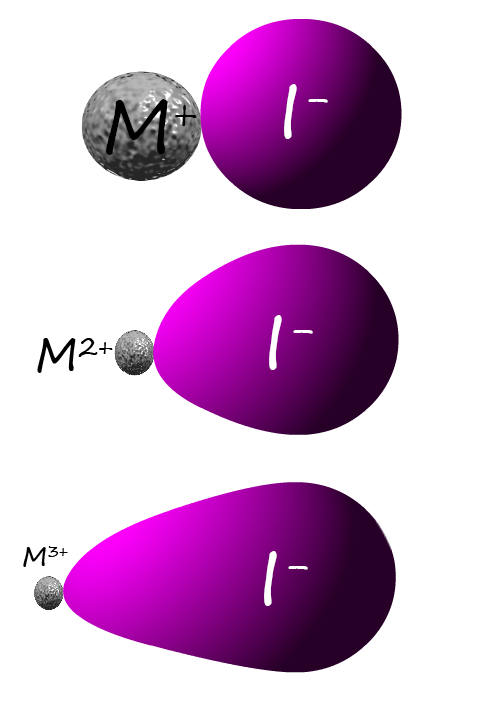 In an ionic compound when the size difference between the
metal cation
and the non-metal anion
is not that different the ions are spherical and this results in the formation of a model
ionic bond.
However as the metal cation get smaller and their charge increases they are able to attract the
electron density around the non-metal anions back towards the metal ion. This results in the
spherical non-metal anions becoming oval or egg shaped, we say they are
polarised (polarised simply means that the electron density around an atom
has been squashed or stretched or distorted in some way). The larger
the non-metal anion and the smaller and more highly charged the
metal cation the more the anion is polarised or distorted.
In an ionic compound when the size difference between the
metal cation
and the non-metal anion
is not that different the ions are spherical and this results in the formation of a model
ionic bond.
However as the metal cation get smaller and their charge increases they are able to attract the
electron density around the non-metal anions back towards the metal ion. This results in the
spherical non-metal anions becoming oval or egg shaped, we say they are
polarised (polarised simply means that the electron density around an atom
has been squashed or stretched or distorted in some way). The larger
the non-metal anion and the smaller and more highly charged the
metal cation the more the anion is polarised or distorted.
As the metal cation attracts the electron density
back towards it from the non-metal anion then the
electron density is in effect being shared between the two ions, this is what we expect in a
covalent bond. What we end up with is
an ionic bond with some degree of covalent character.
The amount of covalent character present in an ionic bond
will depend upon the charge/size ratio
of the metal cation and the size of the
non-metal anion. The diagram opposite uses the large iodide
anion as an example.
As you can see from the image opposite large metal cations with a +1 charge
are not able to distort or polarise the iodide anion
and the spherical anion and cation ions would show that the bonding is ionic, however small metal cations with
large charges such as
Mg2+ or Al3+ will polarise the
large iodide anion and result
in an ionic compound with covalent characteristics.
Covalent characteristics would include: