

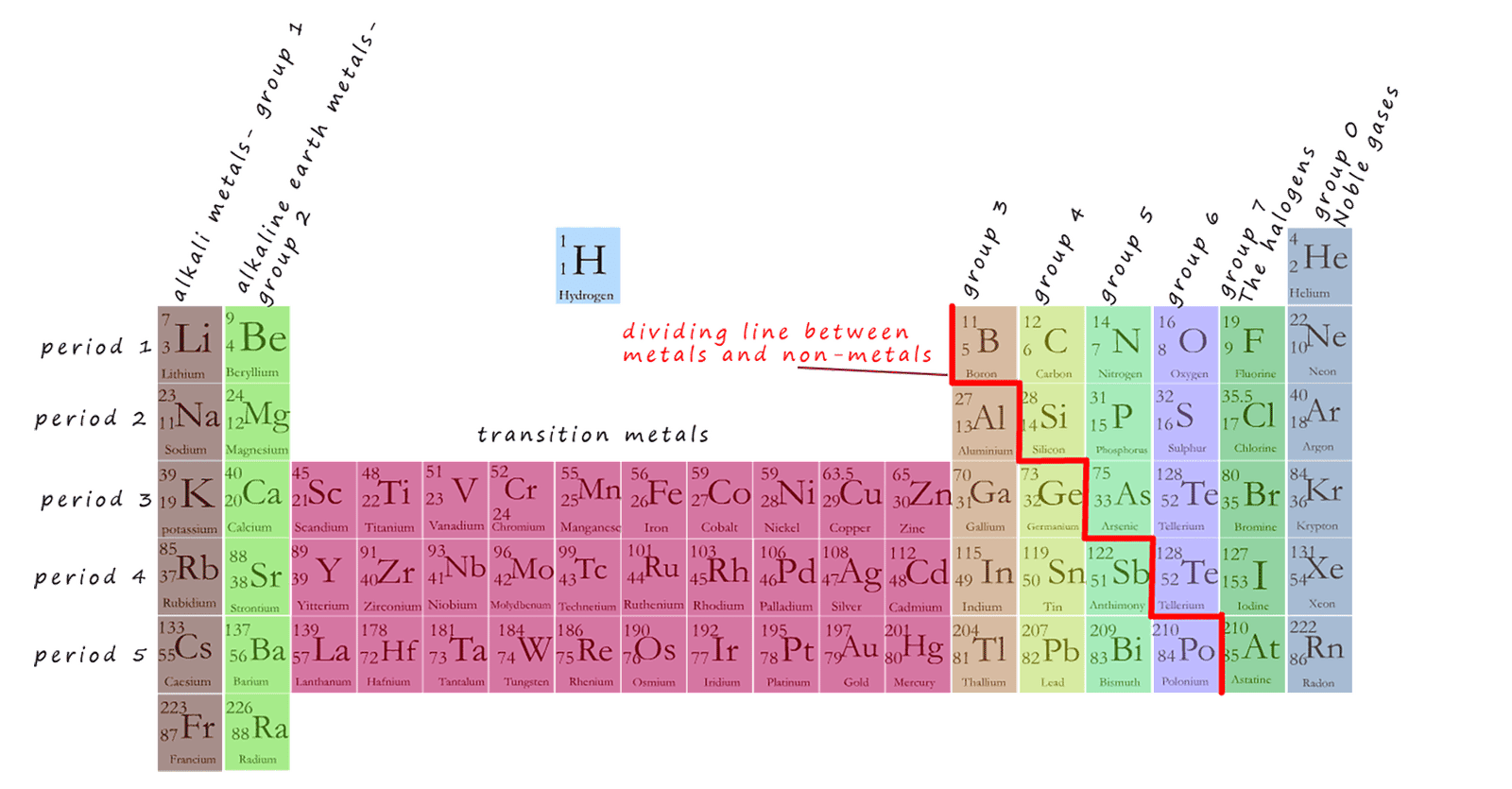
Metals are found in the left hand side of the periodic table in group 1 (the alkali metals), group 2 (the alkaline earth metals) and the top of group 3 as well as in the middle block of the periodic table called the transition metals as outlined in the periodic table shown below:
Ionic compounds are mostly formed when metals from the left-hand side of the periodic table, usually metals from groups I and II react with
non-metal elements on the
right- hand side of the table, usually the halogens from group 7 such as chlorine, bromine and iodine as well as other non-metal elements such as oxygen, sulfur, and phosphorus from groups 5 and 6. The noble gases in group 0 are generally unreactive towards metals due to their high ionisation energies and low affinity for electrons.
The alkali metal sodium for example undergoes a very violent exothermic reaction with the halogen chlorine to produce the ionic compound sodium chloride (NaCl) . This is shown in the image below where a small piece of sodium metal is placed in a flask containing dry chlorine gas. Initially no reaction takes place; but if a few drops of water are placed onto the cube of sodium from the pipette a violent reaction is initiated with the chlorine gas. The flask quickly fills with a white cloud/powder; this white cloud is acutally the ionic compound sodium chloride, this is shown in the image below:
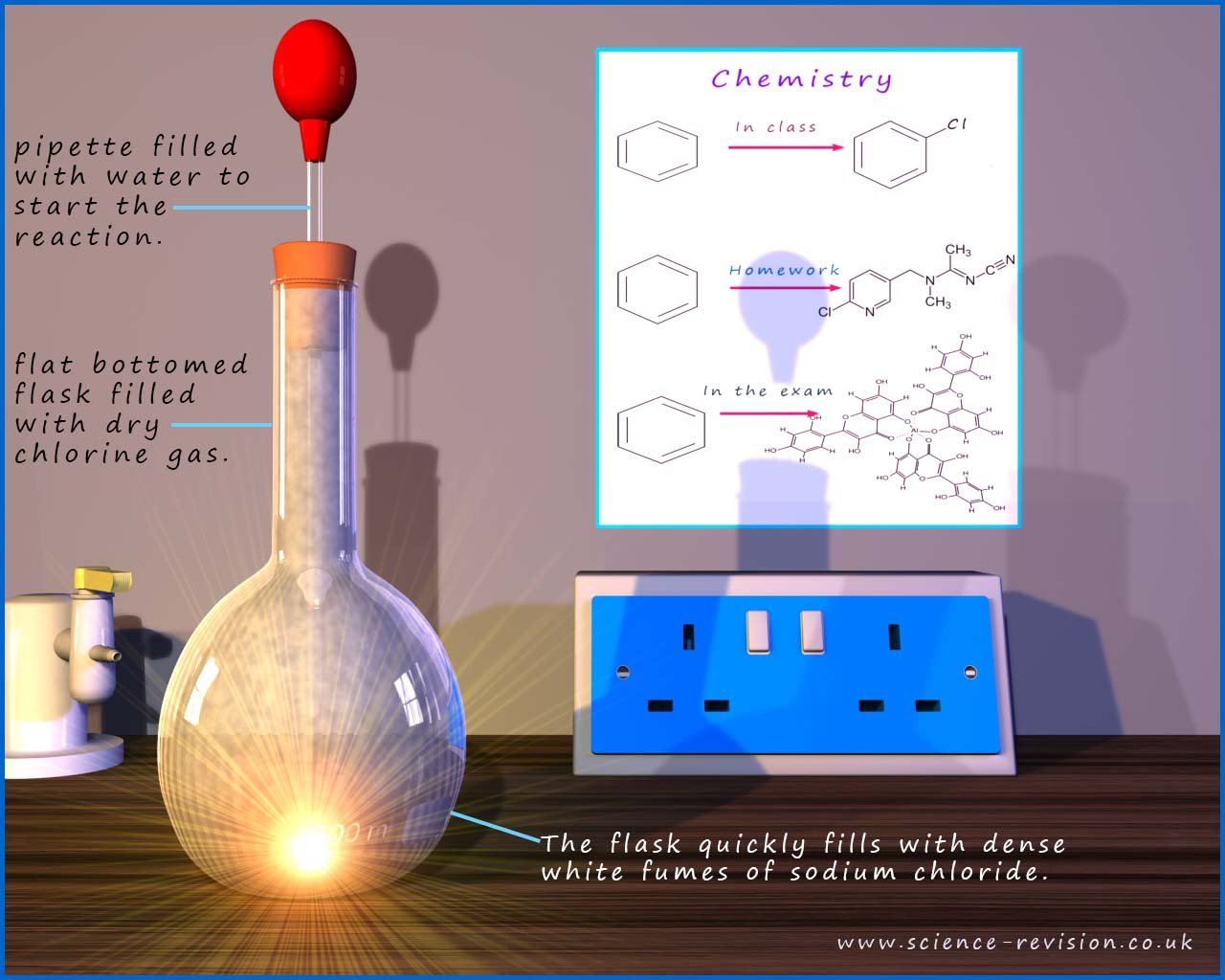
An equation for the reaction is shown below.
 You learned in gcse chemistry that metals when they react tend to lose
electrons to form
positively charged ions called (cations) while
non-metals tend to gain electrons in their reactions and form negatively charged ions called anions. We also used the Octet rule to decide on the number of
electrons lost or gained by an element when it reacts. This simple rule simply states that elements react to achieve the same
electron
arrangement as the nearest noble gas to them in the periodic table; that is to achieve full outer
electron shells e.g.
You learned in gcse chemistry that metals when they react tend to lose
electrons to form
positively charged ions called (cations) while
non-metals tend to gain electrons in their reactions and form negatively charged ions called anions. We also used the Octet rule to decide on the number of
electrons lost or gained by an element when it reacts. This simple rule simply states that elements react to achieve the same
electron
arrangement as the nearest noble gas to them in the periodic table; that is to achieve full outer
electron shells e.g.
The octet rule is a remarkable simple but effective aid in helping us to understand the reactions of many elements however it is a rule and not a law and there are many exceptions to the octet rule which you should be aware of. The octet rule really only works effectively for the first 20 elements or so in the periodic table, once you get to element 21 scandium; you enter the d-block where the octet rule cannot be reliably applied. However despite these exceptions the octet rule is still a useful tool in the chemist's toolbox, albeit a limited one!
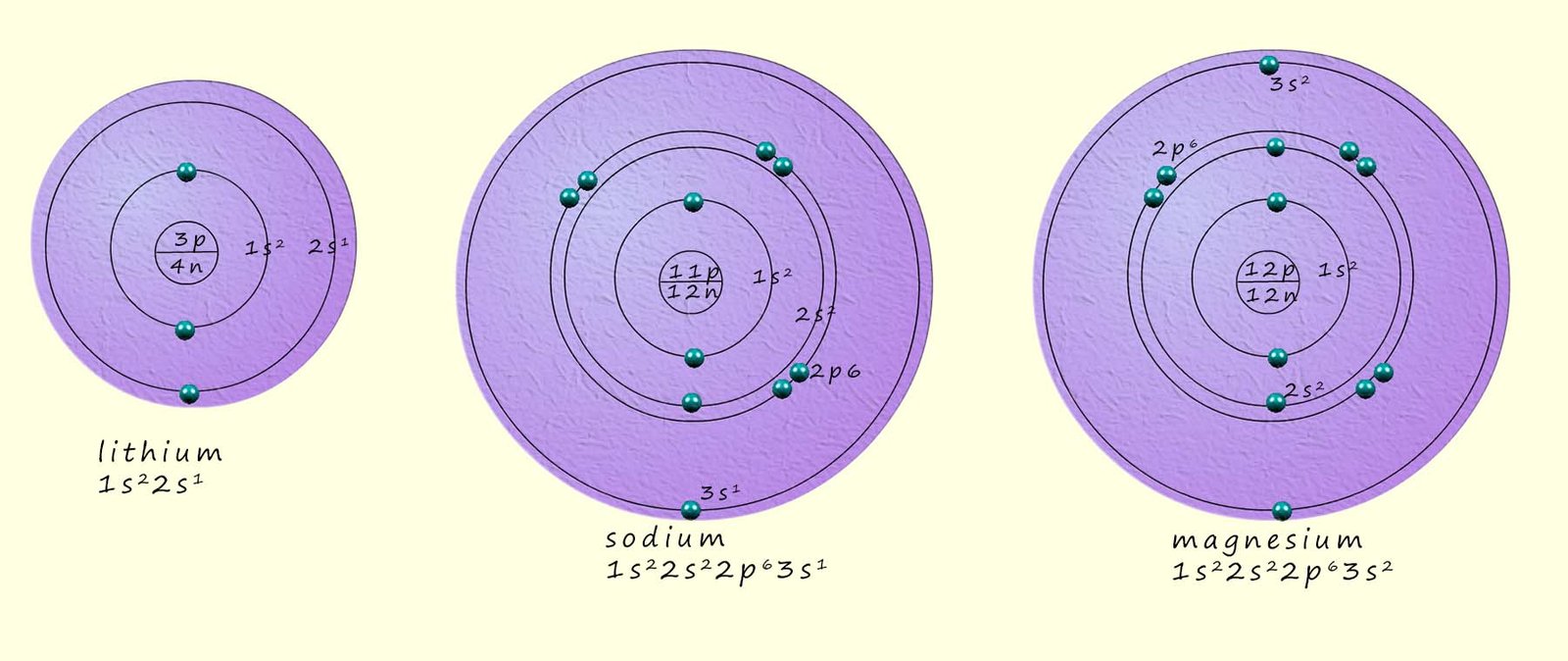
As a simple example of an ionic compound consider the formation of the compound sodium chloride, now sodium is an alkali metal with an
atomic number of 11. Sodium has an electron arrangement of 2,8,1 and an electron configuration of 1s22s22p63s1.
To completely fill its
last shell it needs to gain 7 electrons or simply its outer or valence electron. Obviously it will be easier and
require much less energy to simply lose 1 electron than try and gain 7 electrons. So when
sodium metal reacts as mentioned above it will lose its
outer shell or valence electron.
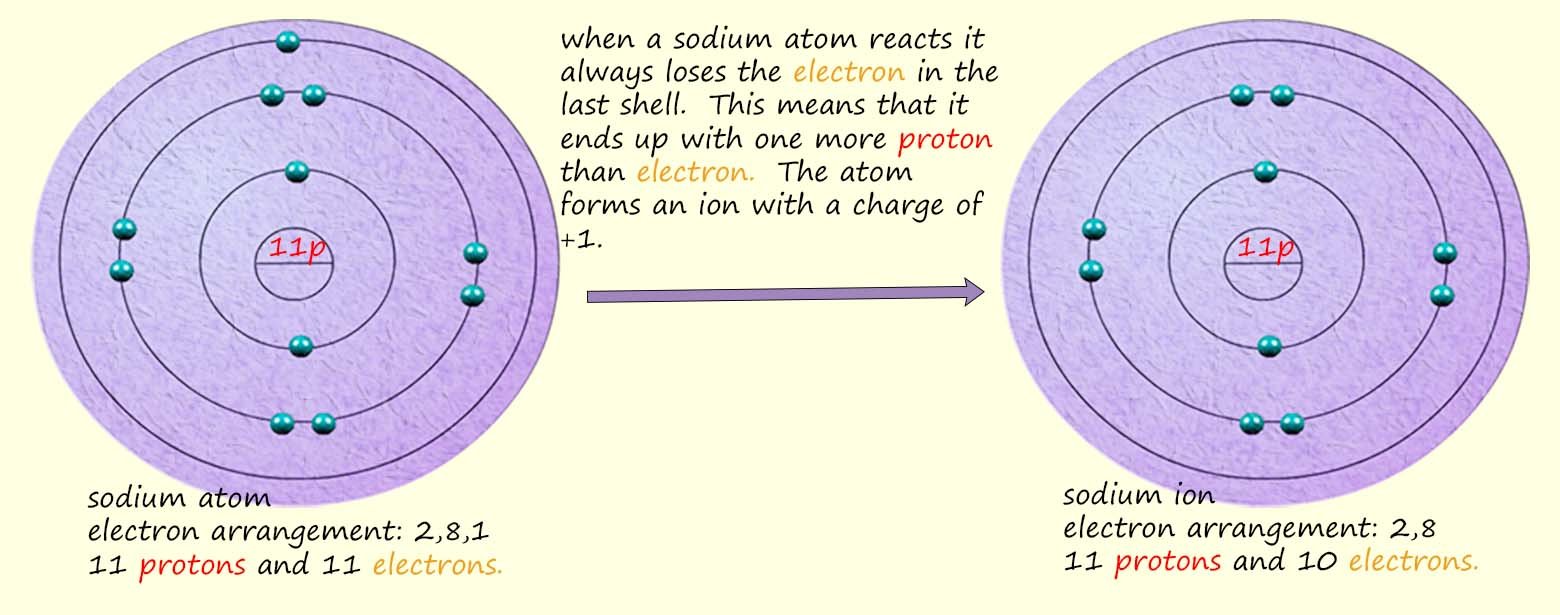
The sodium atom will now have the electronic arrangement 2,8 or
1s22s22p6 the same
as the noble gas neon. It will have 10 electrons. However the nucleus of the sodium atom will still
contain 11 protons with each one having a positive charge. This means that the sodium atom has 11
positive charges (protons) but only 10 negatively charged electrons and so overall the atom has more
positive charges than negative charges to cancel them out. This leaves the
sodium atom with a charge
of +1. Recall that we call atoms with charges ions. Atoms are neutral because they have equal numbers of positively charged
protons in the nucleus and negatively charged electrons in the electron shells but in
ions the numbers
of protons and electrons is NOT equal; so the sodium atom now has a charge- it's now an
ion. Positively charged ions are often referred to as cations and negatively charged
ions are often called anions.
This is shown in the
diagram below
The chlorine atom has an electron arrangement of 2,8,7 or an electron configuration of 1s22s22p63s23p5 this means it only needs to gain 1 electron in order to end up with a noble gas electronic configuration of 3s23p6. So when sodium and chlorine react the chlorine atom will gain the 3s1 electron from the sodium atom to form a chloride ion, a negatively charged anion. This is shown in the diagram below.

The electron from the sodium atom is transferred to the chlorine atom which results in the formation of a positively charged sodium ion (Na+) and a negatively charged chloride ion (Cl-). The diagram below shows the transfer of the electron in the 3s1 sub-level in the sodium atom to the 3p sub-level in the chlorine atom.

In diagram above shows the electron transfer from the 3s sub-shell of a sodium atom to the 3p sub-shell of the chlorine atom. Both atoms end up with the noble gas np6 electronic configuration. In the case of sodium once it loses its outer 3s1 valence electron the ion formed will have an electronic configuration of: 1s22s22p6, the same electronic configuration as the noble gas neon. Chlorine will end up with an electronic configuration of 1s22s22p63s23p6, the same as the noble gas argon.
Magnesium is an alkaline earth metal in group 2 of the periodic table, its electron arrangement is 2,8,2 and its electron configuration is 1s22s22p63s2. To achieve a noble gas electron configuration the magnesium atom needs to lose 2 electrons. This is exactly the same situation as in the sodium chloride example above. The only difference here is that the magnesium atom needs to lose 2 electrons and so it will form an ion with a 2+ charge. So when magnesium reacts with chlorine it will require two chlorine atoms to react with since each chlorine atom is only prepared to accept one electron. The diagram below and also the dot and cross diagram below shows the formation of magnesium chloride and the transfer of 2 electrons from the magnesium atom to 2 chlorine atoms.
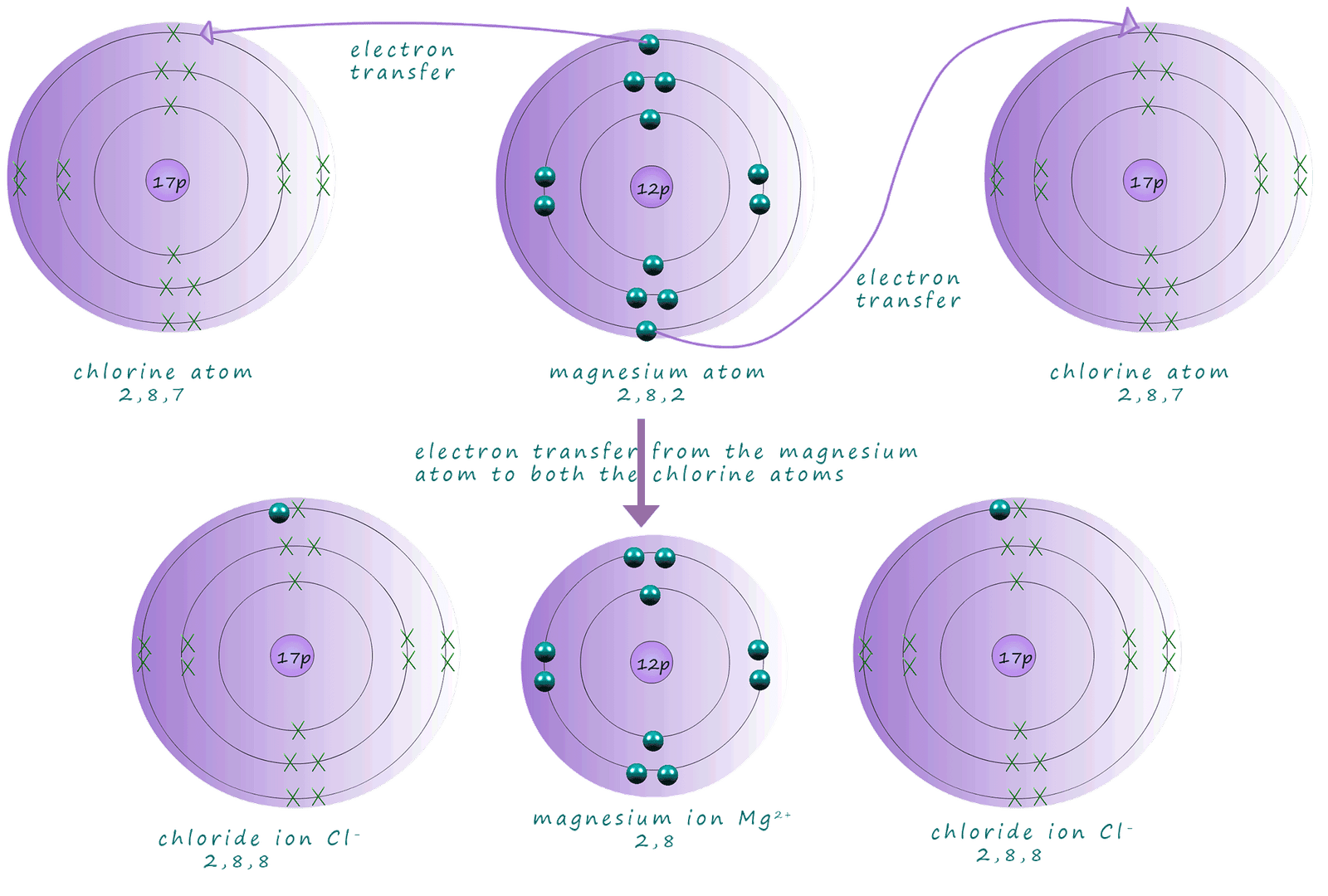
In magnesium chloride each chloride ion has 8 electrons in its valency shell or a 3p6 outer electron configuration; the same as the noble gas argon. The magnesium ion also has 8 outer electrons and full valency outer shell with a 2p6 electronic configuration; the same as the noble gas neon. In gcse chemistry you probably drew dot and cross diagrams to show this electron transfer happening, as shown below:

All ionic compounds have a giant structure composed
of positive and negative ions. The formula of an
ionic compound simply gives the ratio in which the ions are present within the giant lattice. Sodium chloride
for example has the formula NaCl, this simply tells you that the sodium and
chloride ions are present in the
ratio of 1:1 in the giant ionic lattice.
To work out the formula for any compound is very straight forward. You only really need a periodic table to
be able to check what group in the periodic table an element is found in. The group will tell you the number of bonds the element makes.
This will be the number of electrons lost or gained to achieve a stable octet of electrons in the last/valency
shell. This is shown in the table below:
| Group in periodic table | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Charge on ion | +1 | +2 | +3 | -3 | -2 | -1 | 0 | Valency (number of bonds the element makes | 1 | 2 | 3 | 4 | 3 | 2 | 1 | 0 |
Example:
What is the formula for the
ionic compound lithium oxide?:
| element | lithium | oxygen | |
|---|---|---|---|
| element symbol | Li | O | |
| valency | 1 | 2 | |
| swap over the valencies | 2 | 1 | |
| ratio | Li2 | O | |
| formula | Li2O | ||
| The ionic formula is simply the formula with the charges on the ions present. The charges on the ions are simply the number of electrons lost or gained when the element reacts. This is the same as its valency. | |||
| ionic formula | Li2+O2- | ||
If you have worked out the formula correctly the charges on the ions should always balance or cancel out. In this case there are 2 lithium cations, each with a charge of +1. This gives a total positive charge of 2+. This will be cancelled out by the 2- charge on the single oxide ion present. If you need to revise how to work out the formula for substances then click here.
The transition metals can form both ionic and covalent compounds, though the nature of the bonding can vary widely depending on the specific metal and the elements it interacts with, for example transition metals can form ionic compounds, particularly with non-metals such as the halogens (e.g. iron (III) chloride, FeCl3) and oxygen (e.g. titanium dioxide, TiO2). In these compounds, the transition metals typically lose electrons to form positively charged ions (cations).
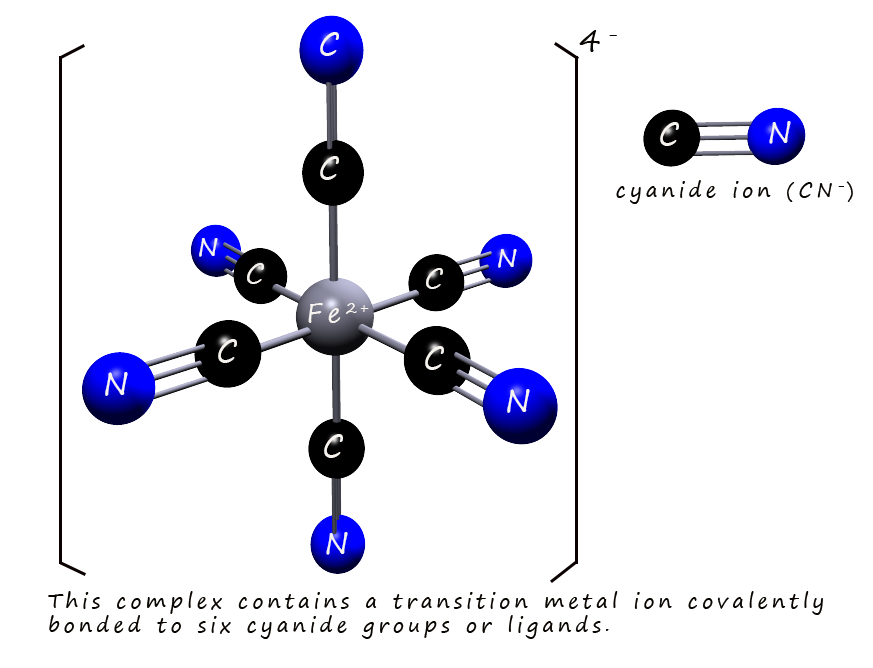
However transition metals also frequently form covalent compounds, especially in coordination complexes which you will study later in your A-level course, in these complexes the transition metals bond with atoms or groups called ligands usually by sharing a pair of electrons in a covalent bond, a ligand is simply a neutral molecule or ion that has a lone-pair of electrons that it can use to form a co-ordinate bond with the transition metal by donating a pair of electrons, for example the transition metal iron can form six covalent bonds to a cyanide ion (CN-) to form a molecule or a complexes such as the one shown in the image opposite, which shows the [Fe(CN)6]4- (hexacyanoferrate(II)) complex clearly showing the six covalent bonds formed by the iron ion. The type of bonding formed by the transition metals depends on a number of factors including the oxidation state of the transition metals, which often have partially filled d orbitals and the nature of the molecule or ion it bonds with which allow for a variety of bonding possibilities.
Most of the discussion above has been about ions and how they are formed, however once you are confident about how these cations and anions are formed then it should be obvious that once formed these positive and negatively charged ions will attract each other by electrostatic forces. Therefore an ionic bond is simply the electrostatic attraction between oppositely charged ions. Ionic compounds consist of giant structure of ions called an ionic lattice. Click on the link below or here for more information on the structure of ionic compounds.