Finding the formula of compounds
Working out the chemical formula for compounds is a valuable skill needed in chemistry. Chemical equations are usually written as either as word or symbolic equations; however symbolic equation are generally more useful than word equations. However in order to write out balanced symbolic equations you will need to be able to work out the chemical formula for compounds; luckily this is very easy to do.
Working out chemical formula
To work out the chemical formula for a compound all you really need is copy of the periodic table. Using the periodic table we can find out what group any element is in and from this we can easily determine its valency; that is how many chemical bonds it will make. An outline of the periodic table is shown below with groups 1-8 labelled.
Using the periodic table to find the valency of an element

Now as we mentioned the number of bonds an element makes it called its valency and the number of bonds an element makes is simply the number of electrons it needs to lose or gain to achieve a stable octet of electrons; that is a full last shell. The valency or number of electrons an element needs to lose or gain to achieve a stable octet of electrons is very easily worked from the periodic table. The metals found in groups 1, 2 and 3 of the periodic table will all lose electrons when they react with other elements for example:
- Group 1 metals such as lithium, sodium and potassium will lose 1 electron; that is they have a valency of 1 or they will form 1 bond.
- Group 2 metals such as magnesium and calcium will lose 2 electrons; that is they have a valency of 2 or they will form 2 bonds.
- Group 3 metals such as aluminium will lose 3 electrons; that is they have a valency of 3 or they will form 3 bonds.
While
This is summarised in the table below:
| group in the periodic table where the element is found | group 1 | group 2 | group 3 | group 4 | group 5 | group 6 | group 7 | group 0 or group 8 |
|---|---|---|---|---|---|---|---|---|
| valency/number of bonds the element makes | 1 | 2 | 3 | 4 | 3 | 2 | 1 | 0 |
The valency of the non-metal elements
Now groups 4, 5, 6, 7 and 0 in the periodic table contain the non-metal elements and when these elements react unlike the metal which lose electrons when they react the non-metals are looking to gain electrons to complete their octet of electrons; for example:
Self-check
- Group 4 non-metals such as carbon or silicon will gain 4 electrons to achieve an octet of electrons in their outer shell; that is they have a valency of 4 or they will form 4 bonds.
- Group 5 non-metals such as nitrogen or phosphorus will gain 3 electrons to achieve an octet of electrons in their outer shell; that is they have a valency of 3 or they will form 3 bonds.
- Group 6 non-metals such as oxygen or sulfur will gain 2 electrons to achieve an octet of electrons in their outer shell; that is they have a valency of 2 or they will form 2 bonds.
- Group 7 non-metals such as fluorine, chlorine or bromine will gain 1 electron to achieve an octet of electrons in their outer shell; that is they have a valency of 1 or they will form 1 bonds.
- Group 0 non-metals such as helium, neon or argon are all unreactive gases that do not easily react since they already have full outer electron shells; they therefore do not readily form bonds with other elements.
And finally
The table above summaries the link between the group in the periodic table an element is found in and its valency and using this simple table it is possible to work out the molecular formula for many different compounds.
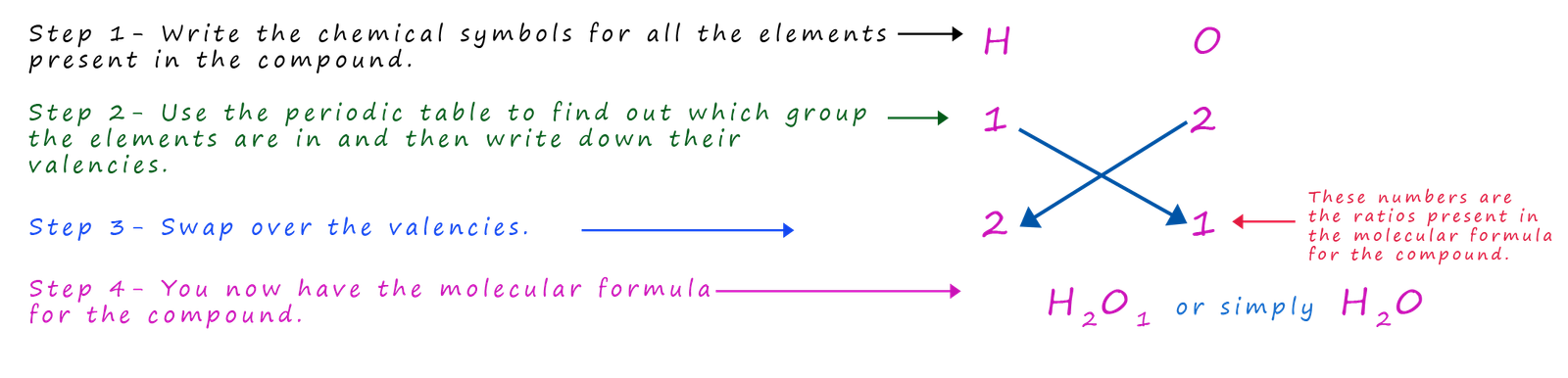
Example 1- hydrogen oxide (water)

As a simple example let's start with a chemical formula
that everyone knows- H2O; the molecular formula for a molecule
of water. The image opposite shows a molecule of water; we can see that the oxygen atom is making two bonds; that
is its valency is
2 while each hydrogen atom makes only 1 covalent bond; so its valency is one.
Let's imagine for a minute that you did not know the molecular formula for water; how would you work it out? The method
shown below is often called the cross-over method for obvious reasons and by simply following a few simple steps you can easily work out the
chemical formula for
water.
UUsing this simple method it is possible to work out thechemical formula for almost all the compounds you are likely to meet in your chemistry course.
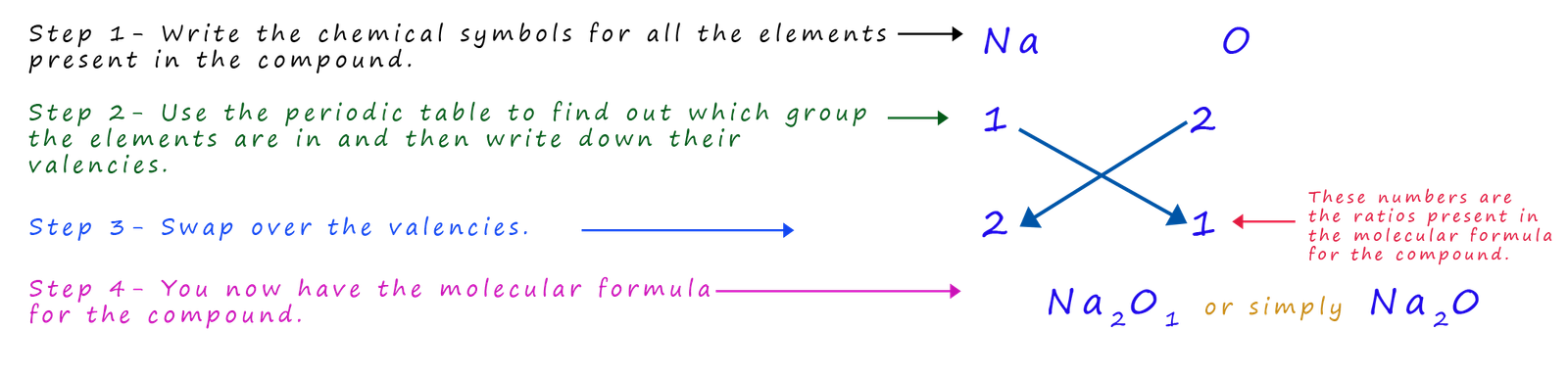
Example 2- sodium oxide
Almost everyone knows the chemical formula for water, but if you asked someone for the chemical formula of say sodium oxide the chances are they probably won't know it. However it is easy using the method above to work out its formula:
Example 3-Phosphorus chloride
What is the formula for the compound formed when phosphorus reacts with an excess of chlorine gas? Well just follow the method above to get the formula for this compound:

Example 4- calcium sulphide
Calcium an alkaline earth metal in group 2 reacts with the non-metal sulfur in group 6 to form calcium sulfide. What is the formula for calcium sulfide?
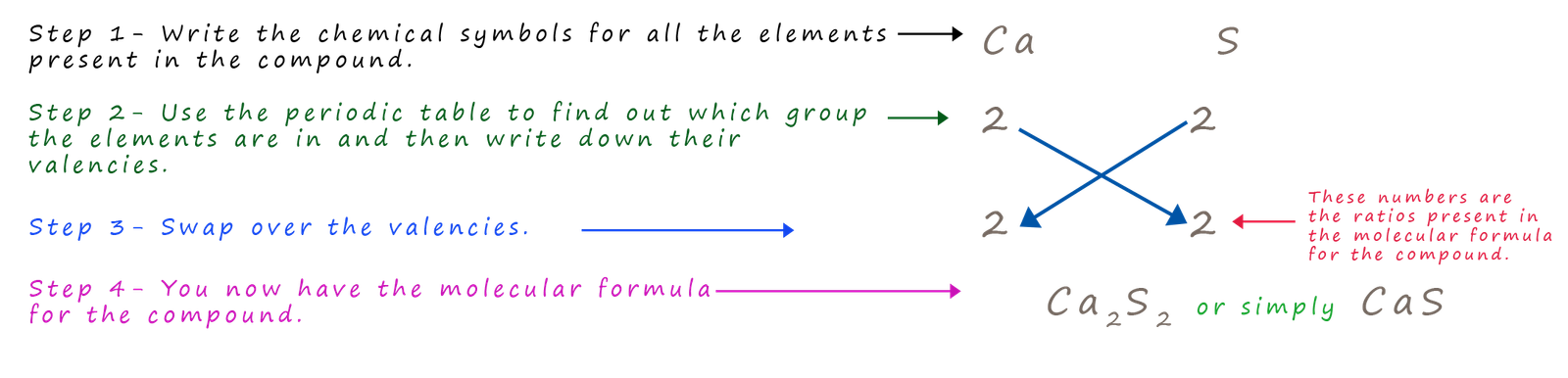
In this example there is one additional step needed. When the two numbers in the formula are multiples of each other, such as 2 and 4 or 3 and 6 or in this case 2 and 2 we simply cancel down to get the simplest ratio possible for the elements. In this case simply divide by 2 and Ca2S2/2 simply becomes CaS
Finding the formula for compounds containing more than two elements

All the compounds we have worked out the formula for so far have all had two elements in them, compounds containing only two elements ends in the letters -ide. However you will no doubt have met other compounds which contain more than two elements and which have different ending to their names e.g.
- Some compounds end in the letters -ate. This generally means that the compound contains at least three elements one of which is oxygen e.g. potassium permanganate is a dark purple solid with the formula KMnO4. This compound; shown in the image opposite; contains three elements: potassium (K), manganese (Mn) and oxygen (O).
- A common alkaline solution you will have used in your chemistry lessons is sodium hydroxide. This compound is made up of three elements: sodium (Na), hydrogen (H) and oxygen (O).
- Another less common ending found in some compounds is -ite e.g. sodium sulfite.
The formula of multiple element compounds
So how do we go about working out the formula for these compounds that contain more than two elements? Well we use the same basic method as above but it is necessary to change it slightly. Many of these compounds which contain multiple elements contain common group ions. You will have met these group ions before and probably not realised it. If you have used compounds which have the following names then these compounds all contain these group ions:
- hydroxide (OH-) ions
- sulfate (SO42-) ions
- nitrate (NO3-) ions
- phosphate (PO43-) ions
- ammonium (NH4+) ions
- carbonate (CO32-) ions
Group ions
Many of the compounds you will meet in chemistry contain these group ions and you should make an effort to learn their names and formula. The most common group ions you are likely to meet are shown below:

So how do we go about finding the formula for compounds which contain these group ions? As an example consider sodium sulfate. Now sodium sulfate contains sodium ions and sulfate ions; we can easily find the valency of sodium ions. Sodium is a group 1 alkali metal found in the first group in the periodic table so its valency is 1. However sulfate is a compound and obviously will not be found in the periodic table of elements, so how do we find out how many bonds it makes? What is its valency? That's the easy part, it's valency is simply the charge present on the ion. The sulfate ion has the formula SO42-, its charge is simply 2-; so its valency is the same as its charge; that is 2. To find the formula of sodium sulfate simply use the cross-over method from above:
Example 5 - Sodium sulfate

As another example of a compound containing one of these group ions consider the alkali calcium hydroxide:
Example 6- Calcium hydroxide


You will have noticed that in writing the formula for calcium hydroxide it was necessary to include a set of brackets. Without the presence of these brackets the formula would be wrong e.g. when we worked out the formula for calcium hydroxide using the cross-over rule above it should be noted that calcium hydroxide contains ONE calcium ion and TWO hydroxide ions. That is:
- Ca
- OH
- OH
CaOH2 then this would be wrong, since it gives us 1 Calcium ion, 1 oxygen and 2 hydrogen atoms. This is not what we want! We want 1 calcium ion and TWO hydroxide ions. So the only way to do this is to have the hydroxide ion in brackets to indicate how many of these ions we need. So when we write the formula as Ca(OH)2 the "2" after the hydroxide ion means that everything inside the brackets is multiplied by 2, or put another way it means we have TWO hydroxide ions.
Example 7- Magnesium phosphate
The phosphate ion is a group ion with the formula PO43-, it has a valency of 3. Magnesium is a group 2 metal and so has a valency of 2. We can find the formula of magnesium phosphate using the method above:

Magnesium phosphate has 3 magnesium ions and two phosphate ions in its formula. Imagine how the formula would read if we did not use brackets, we would have Mg3PO42, that is 3 magnesium ions, 1 phosphorus atom and 42 oxygen atoms- not what we want!

Finding the formula for compounds containing transition metals
Many of the compounds you will use in your chemistry course will contain transition metals, for example you will no doubt have used one or more of the following compounds while carrying out practical work in the lab: copper sulfate, sodium dichromate or potassium permanganate. Working out the formula for a compound containing a transition metal might seem impossible since the transition metals are found in the middle block of the periodic table and so there is no way you can work out their valencies.
However if you look at the names of many transition metal compounds you will see Roman numerals after the name of the of the transition metal present in them e.g. iron(III) oxide, copper(II) chloride or copper(I) oxide. These Roman numerals are the valencies of the transition metals present in the compound; that is the number of bonds the transition metal is making in the compound. For example what is the formula for copper (II) sulfate?
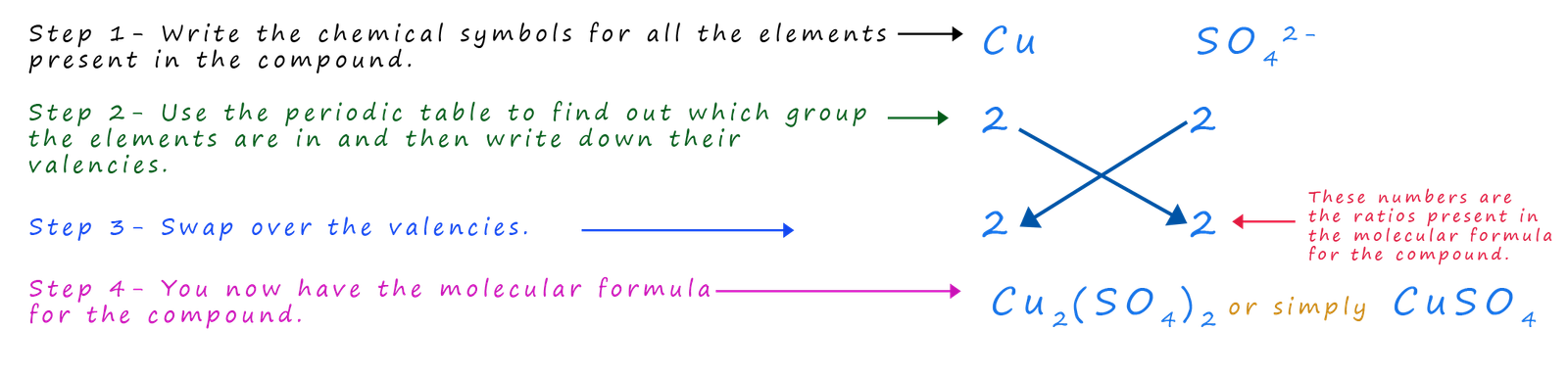
Finding the formula for acids and alkalis
All acids contain hydrogen ions (H+) in their chemical formula. The other ion present in most acids are the group ions such as sulfate, nitrate and phosphate. Working out the molecular formula of an acid is very straight forward e.g. what is the molecular formula for sulfuric acid? Like all acids; sulfuric acid contains (H+) ions and it also contains the sulfate ion (SO42-), so we simply use the method above to work out its molecular formula:
What is the formula for sulfuric acid?
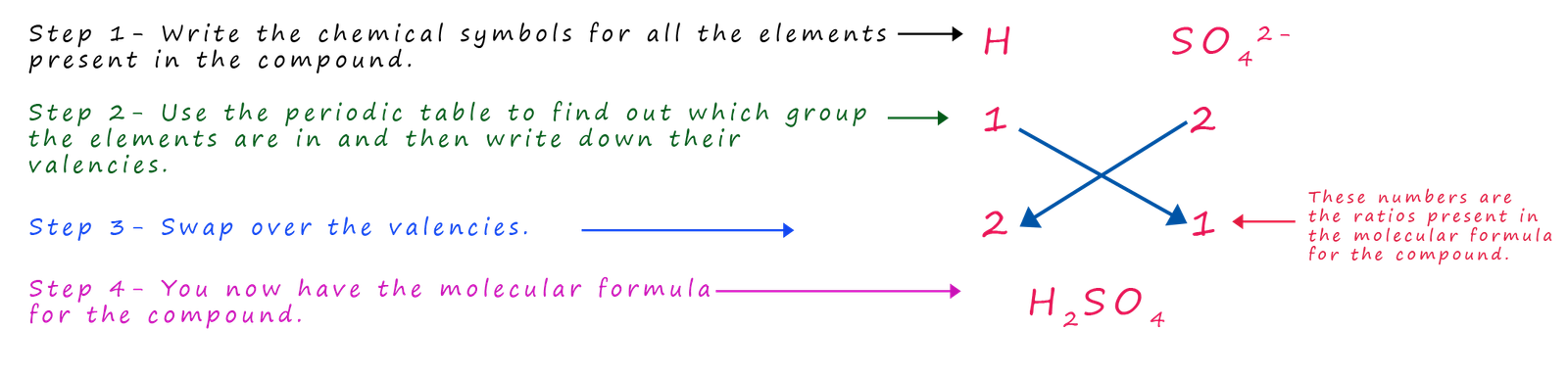
While sulfuric acid contains hydrogen ions (H+) and sulfate ions other acids such as nitric acid, phosphoric acid and carbonic acid all contain hydrogen ions (H+), as do all acids, but they contain different group ions e.g.
- Nitric acid contains hydrogen ions (H+) ions and nitrate ions (NO3-); this will give a formula for nitric acid of HNO3
- Phosphoric acid contains hydrogen ions (H+) ions and phosphate ions (PO43-) ions; this will give a formula for phosphoric acid of H3PO4.
- Carbonic acid contains hydrogen ions (H+) ions and carbonate ions (CO32-) ions; this will give a formula for carbonic acid of H2CO2.
What is the formula for the alkali sodium hydroxide?
Sodium hydroxide contains sodium ions and hydroxide ions (OH-), the formula for sodium hydroxide is simply:
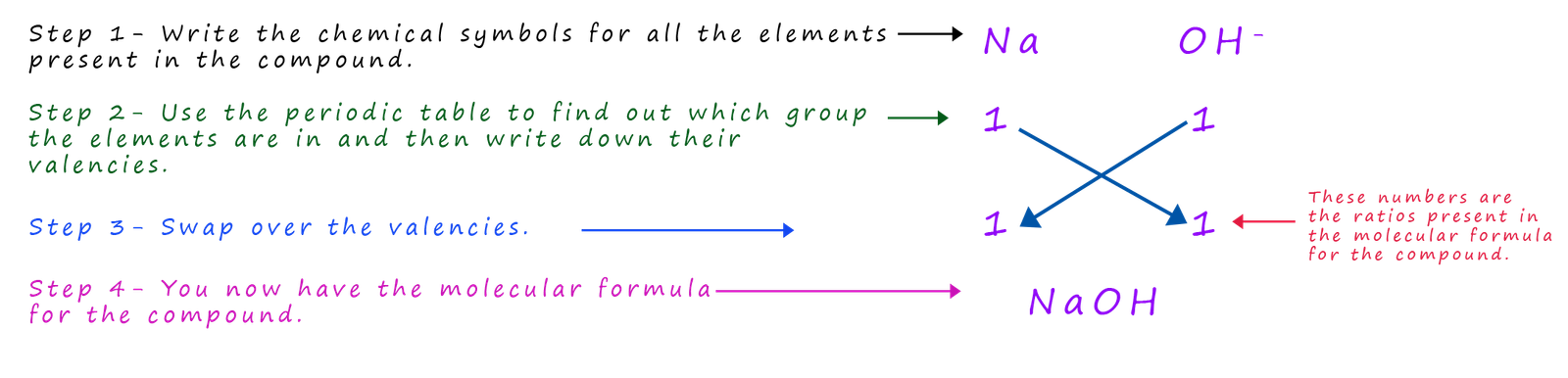
And for the alkali calcium hydroxide we have:

Key points
- The valency is the number of bonds an element makes.
- The valency for an element is found from its position in the periodic table. It is related to the number of electrons the element needs to complete an octet of electrons in its outer electron shell.
- Group ions are compounds which are found in many chemical substances. The charge on the group ion is the same as its valency.
Finding the Chemical Formula
Practice questions and finding the formula activity
To review your understanding on how to work out the chemical formula of compounds try working out the formula for the compounds in the activity opposite or answer the questions in the worksheets below, simply click the links to access the worksheets:

