


A mass spectrometer is used to identify unknown substances, to determine the composition of mixtures and to
accurately measure the relative amounts and masses of substances present. It has many uses in the chemical, engineering
and space industries, the Rovers which NASA sent to the planet Mars all had mass spectrometers on board to
analysis Martian rocks, minerals and gases in the atmosphere.
In the A-level chemistry specifications there are two main types of mass spectrometers that you will need to
know about. If you are studying the AQA specification you will need to be able explain the working of a time
of flight mass spectrometer and carry out some calculations based on this method of mass spectroscopy.
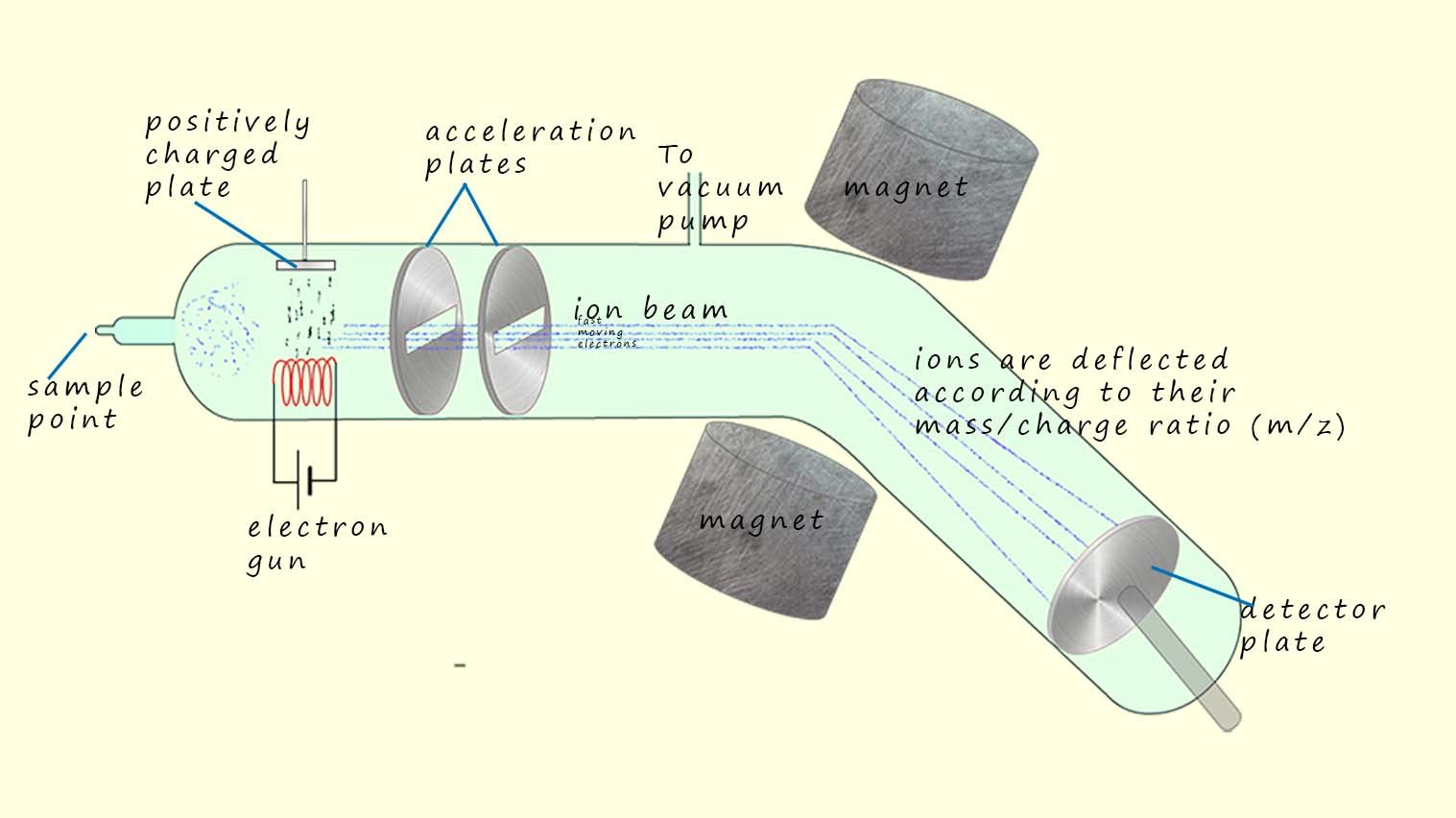
Perhaps the most common mass spectrometer in use is the single beam mass spectrometer. To analyse the sample of the unknown substance under examination we need to consider what happens in each of 4 separate stages in the operation of this type of mass spectrometer. These 4 stages are:
Ionisation is one of the key steps that happen inside the mass spectrometer. In this step the sample atoms or molecules will be turned into ions with a +1 charge. First the sample atoms or molecules are placed into the mass spectrometer at the sample point. A common method used to insert the sample is the use of a Direct Insertion Probe. This method involves placing a solid sample directly into a heated probe, which vaporizes the sample and introduces it into the instrument. Now the sample must be in the form of a gas, so any liquid or solid samples are vapourised before being inserted into the mass spectrometer.
Next this vapour is ionised by being bombarded by fast moving electrons from an electron gun. The image below shows how electron impact ionisation works. Here an electron gun, which is essentially just a wire that is heat to a very high temperature, at this high temperature it emits a steady stream of high energy fast moving electrons. These fast moving negatively charged electrons are attracted to a positively charged plate opposite the electron gun. This positively charged plate will attract and accelerate the electrons given off by the electron gun.
If we imagine the electrons as being like fast moving solid particles or balls/bullets, then when the molecules
or atoms in the sample meet these fast moving
electrons, they will have 1 or more of their outer
electrons removed or knocked off. This will leave a positively charged ion behind.
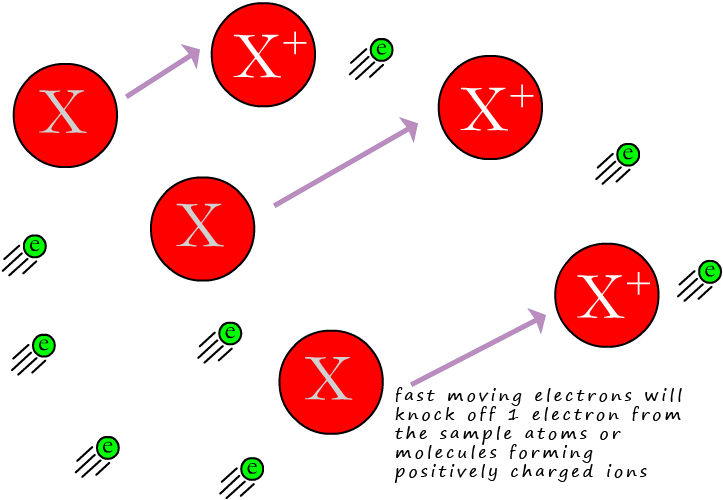 If we call the inserted sample X, then we can show this as:
If we call the inserted sample X, then we can show this as:
Where e represents the electron being knocked off the sample x. You may also see this equation written as:
Here the first e represents the electron from the electron gun which impacts the sample X, forming a positively charged ion, X+(g) and the 2e on the product side represent the one electron lost by the sample X and also the electron from the electron gun.
For example the equations below represent the ionisation of neon gas and hydrogen gas:
or simply:
And for hydrogen gas we have:
or simply:
Since the sample molecules are effectively being bombarded by fast
moving electrons from the electron gun it
is likely
that the sample molecules will be fragmented
or broken up into smaller fragments which will
appear in the mass spectrum of the molecule.
It is also possible that ions with a 2+ charge maybe produced during
this ionisation stage. Though the energy required to remove
2 electrons from the sample molecule
will obviously be much larger than that required to remove a single electron, so these
doubly charged ions will be present in much smaller amounts.
The next key step that occurs in the mass spectrometer is acceleration of the ions formed. The positively charged ions will be accelerated up the tube by an electric field; they will be accelerated towards negatively charged plates. Here they will pass through a series of slits and a single ion beam will form.
One of the main pieces of information that the mass spectrometer provides is the Ar or Mr of the substance under analysis. If the sample contains a mixture of substances it would be very useful if we could obtain the Mr for each substance present. Now after the ionisation step we may have a range of different substances most of these will be ions with a charge of +1. The mass spectrometer will give you a read out of the mass to charge ratio, often called m/z ratio of any substance it detects. Now if the charge (z) is simply equal to +1, then the mass to charge ratio (m/z) is simply the mass.
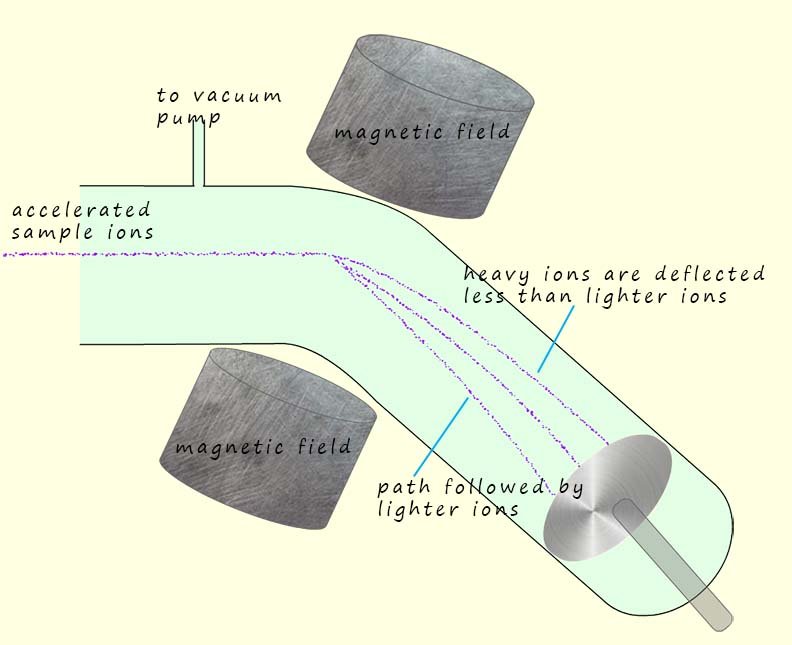
The deflection of ions based on their mass to charge ration (m/z) is perhaps the crucial part in this type of mass spectrometer. The ions are deflected by the strong magnetic field produced by the electromagnet. The amount the ions are deflected depends upon their mass to charge ratio (m/z). The deflection is greatest when:
The detector plate has a negative charge due to excess electrons being present on it. When the positively charged ions arrive at the detector plate they gain an electron and are reduced. This involves the movement of electrons, or a flow of current. The more electrons that flow the more ions are present that is their abundance is high. This whole process will be monitored and controlled by a computer.
You should be able to describe in detail what happens in each of these 4 key stages or steps in the mass spectrometer.