

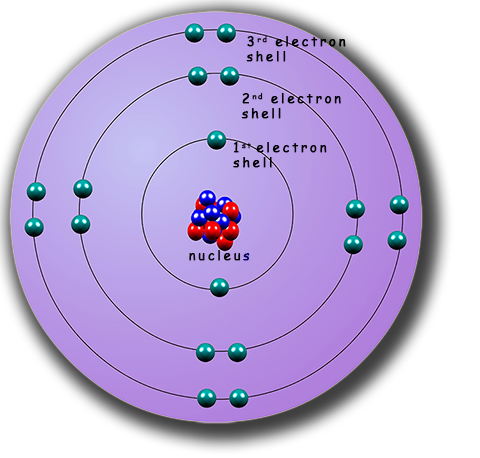
 The model we use to describe the atom is often called the nuclear atom.
In this model of there
is a very small dense nucleus at the centre of the atom, inside which are the
protons and neutrons. The nucleus is very
dense and most of the
mass of the atom is found here. The electrons in an
atom orbit the
nucleus in a series of shells or energy levels.
Atoms are very small and have a radius
of around 1 x 10-10m (0.0000000001m) or 0.1nm ( 1 nanometre= 1 x 10-9m), about 1 million atoms
stacked end to end would be about as thick as a human hair!
The model we use to describe the atom is often called the nuclear atom.
In this model of there
is a very small dense nucleus at the centre of the atom, inside which are the
protons and neutrons. The nucleus is very
dense and most of the
mass of the atom is found here. The electrons in an
atom orbit the
nucleus in a series of shells or energy levels.
Atoms are very small and have a radius
of around 1 x 10-10m (0.0000000001m) or 0.1nm ( 1 nanometre= 1 x 10-9m), about 1 million atoms
stacked end to end would be about as thick as a human hair!
Elements are simple substances which consist of only 1 type of atom; all
known elements are listed in the
periodic table. The elements in the periodic table are arranged in order of
their atomic number (symbol Z), that is the number of protons
in the nucleus. Since
atoms are electrical neutral the number of protons
is the same as the number of electrons. To calculate the number of
neutrons you simply take the atomic number from the
mass number
of the element.
The masses of protons are
neutrons are
almost identical and are shown in the table below. It is the mass of these two sub-atomic particles that make up almost all the
mass of an atom, since the
mass of an electron is negligible when compared to the masses of the proton
and neutron. If you divide the mass of the proton by the mass
of the electron given in the table below it will tell you just how much
more massive the proton is when compared to the electron. The particles
inside the nucleus (the protons and
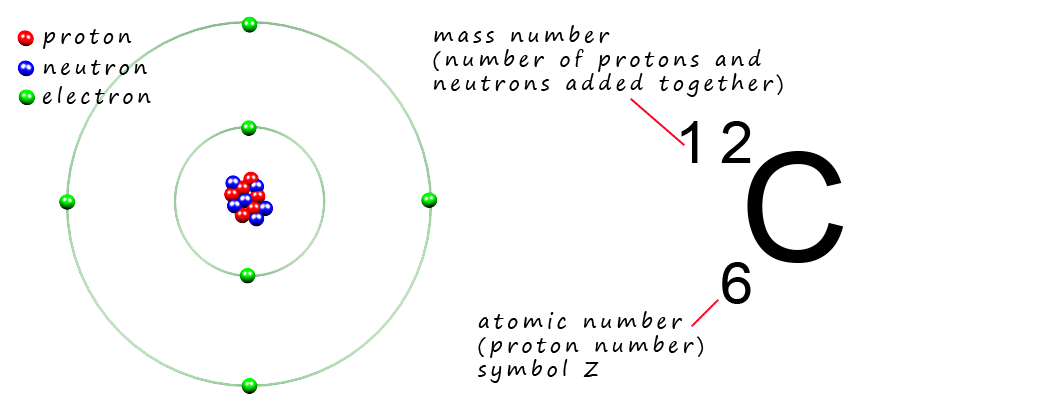
neutrons) are often referred to as nucleons. The diagram below should be familiar to you and shows the
atomic structure of an atom of the element
carbon along with its atomic number and mass number.
| particle | relative mass | actual mass in Kg | charge | Where in the atom it is found | |
|---|---|---|---|---|---|
| proton | 1 | 1.67 x 10-27 | +1 | nucleus | |
| neutron | 1 | 1.67 x 10-27 | 0 | nucleus | |
| electron | 0 | 9.11 x 10-31 | -1 | electron shells or rings |