

 The idea of atoms is not a new one! The Greeks philosopher Democritus suggested over 2500 years ago
that matter was made up of solid tiny balls called atoms. Democritus thought of atoms as unbreakable
spheres. These ideas about atoms changed little for thousands of years and it is really only in the last
120 years or so that any real insight was made into the nature and structure of atoms. One of the first
pioneers on this journey was John Dalton.
The idea of atoms is not a new one! The Greeks philosopher Democritus suggested over 2500 years ago
that matter was made up of solid tiny balls called atoms. Democritus thought of atoms as unbreakable
spheres. These ideas about atoms changed little for thousands of years and it is really only in the last
120 years or so that any real insight was made into the nature and structure of atoms. One of the first
pioneers on this journey was John Dalton.
Dalton was born in 1766 in the Lake District; England.
He was interested in the sciences and in particular chemistry, physics and meteorology. Dalton is best
known for his atomic theory and his gas laws, which you will probably meet in your physics course.
According to Dalton's atomic theory:
Sir John Joseph Thomson was professor of physics at Cambridge University in 1884. Thomson is credited with the discovery of the electron. The idea that atoms were not made of indestructible solid balls had been around for around 30 years or so and the idea of electrons had been around since 1874 when G.J Stoney suggested the name to explain some observations in his work. But Thomson is credited with providing proof of the existence of the electron.
Gases do not normally conduct electricity; however Thomson was investigating how their conductivity changed at very low pressures when very high voltages were applied across them. He set up an experiment similar to the one shown below. It basically consists of a glass tube filled with a gas at very low pressure. There is a fluorescent screen at one end of the glass tube. Half way up the tube were 2 charged plates; one positively charged and one negatively charged. At the other end was a piece of metal which was connected to the negative terminal of a power supply; this was the cathode. A metal disc with a slit in it was connected to the positive terminal of the power supply. A diagram of his apparatus is shown below.
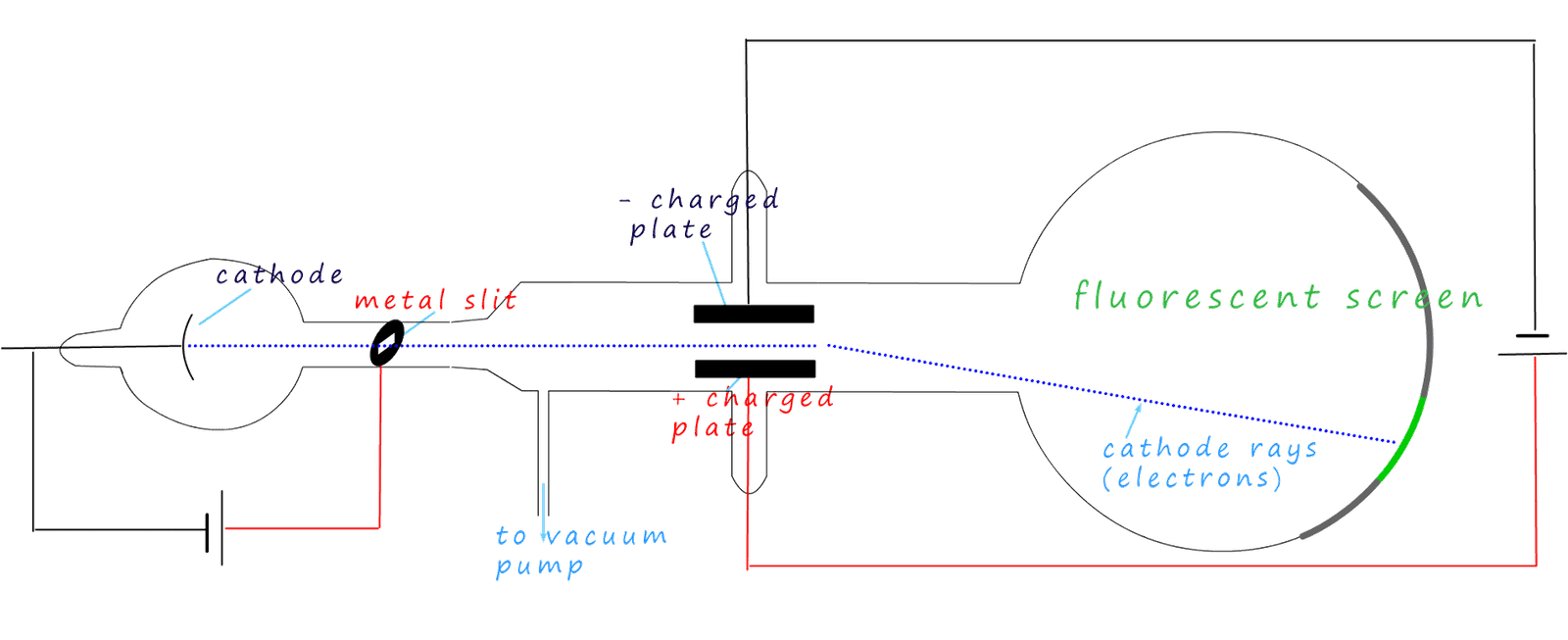
 What Thomson observed while running the experiment was a green glow from the fluorescent screen
where a "beam" or "ray" (shown as blue dots in the diagram) coming from the cathode had struck it.
He noticed that the "ray" was deflected or bent away from the negatively charged plate.
This told him that the "ray" had a negative charge. Since these rays were coming from the
cathode he called them cathode rays. Calculations showed that these cathode rays were over
1840 lighter than a hydrogen atom and that no matter which gas was placed in the tube or if a different
metal was
used as the cathode they
were always produced.
What Thomson observed while running the experiment was a green glow from the fluorescent screen
where a "beam" or "ray" (shown as blue dots in the diagram) coming from the cathode had struck it.
He noticed that the "ray" was deflected or bent away from the negatively charged plate.
This told him that the "ray" had a negative charge. Since these rays were coming from the
cathode he called them cathode rays. Calculations showed that these cathode rays were over
1840 lighter than a hydrogen atom and that no matter which gas was placed in the tube or if a different
metal was
used as the cathode they
were always produced.
Thomson realised that these "corpuscles" as he called them had a
negative charge and they were present in all atoms. So atoms were not solid indestructible
balls after all. The name "corpuscles" was later changed to the electron.
Thomson developed a new model of
the atom to explain his observations. Thomson's model of the atom is often called the
plum pudding model. His idea of what an atom looks like is a sphere of
positive charge
in which are embedded the electrons, a bit like a plum pudding or chocolate chip cookie.
Thompson's calculations had shown that the electron was 1/1840th the mass of a hydrogen
atom.
One of Professor Thomson's students at Cambridge University was the brilliant scientist Ernest Rutherford. In 1911 Rutherford with help from two other research scientists; Hans Geiger and Ernest Marsden conducted a now very famous experiment which led to the idea that atoms contain a nucleus and proved that the plum pudding model proposed earlier by J.J. Thomson was wrong. An outline and details of Rutherford's gold foil experiment is shown in the diagram below.
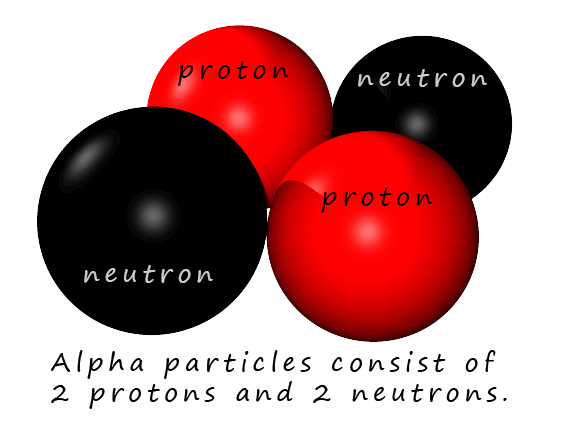
A sample of radioactive radium metal was placed inside a lead block with a hole at one end. Radioactive radium metal emits alpha particles. Alpha particles are large heavy slow moving particles with a charge of 2+, they consist of 2 protons and 2 neutrons. Alpha particles are similar to the nucleus of a helium atom. Alpha particles are only able to travel a few centimetres in air so the whole experiment was carried out in a sealed container in which the air was sucked out by a vacuum pump. Next the alpha particles which were emitted by the radium travelled towards a very thin sheet of gold foil.
Rutherford expected the alpha particles which were fired at the gold foil to simply pass straight through. The set-up Rutherford used is shown below. He was able to detect the alpha particles as they emitted a glow when they struck the fluorescent screen shown in the image below. From the image you can see that most of the alpha particles travelled straight through the gold foil but some were deflected through small angles and some even bounced straight back; as if they had hit something solid and heavy!

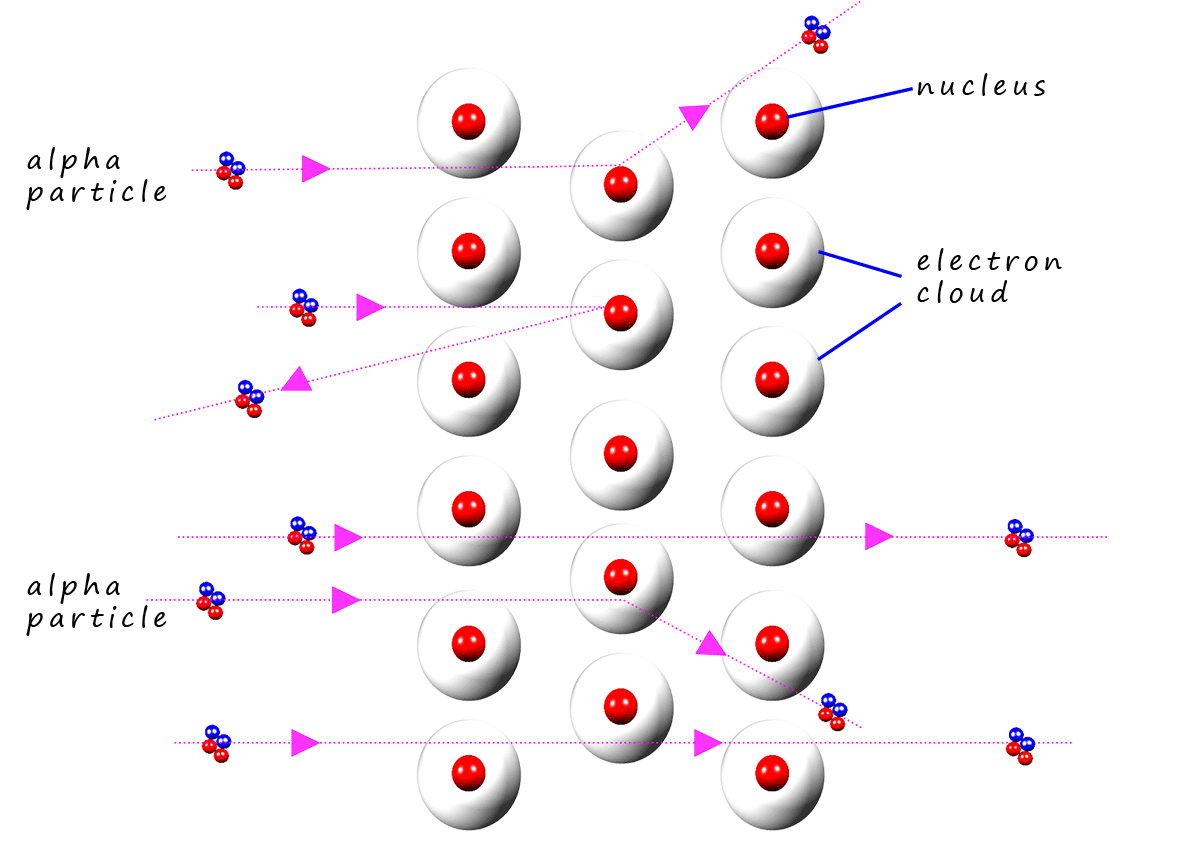
According to Thomson's plum pudding model of the atom the positive charge of the "pudding" was spread out over the whole atom and it would not have been able to stop a heavy bullet like projectile such as a heavy positively charged alpha particle. So Rutherford would have expected all the alpha particles to pass straight through the gold foil. Indeed most of the alpha particles did but some were deflected and some bounced straight back. This was not what Rutherford was expecting at all. This should have been impossible based on the plum pudding model!
Since most
of the alpha particles passed straight through the
gold foil (as shown opposite) and only a few were deflected through large angles,
it was concluded that the atom consisted of mostly empty space but with a tiny area of
positive charge located at the centre of the atom- the nucleus!
The alpha particles that were deflected were those that had come close to the
nucleus; since the
alpha particles
have a positive charge the closer they got to the
positively charged nucleus the greater the angle through
which they would have been deflected (this is shown in the image opposite).
The fact that very few alpha particles; about 1 in 10 0000 were deflected straight back means that the chance of any hitting the
nucleus must have been small; so the nucleus must be tiny.
Rutherford was also able to calculate the size of the nucleus
from his experimental work and he calculated
that the nucleus was about 1/10 000th the size of the atom.
The results from Rutherford's gold foil experiment proved beyond any doubt that Thomson's Plum Pudding model was wrong. However it still left some questions unanswered. The main problem was the electrons. In Rutherford's model of the atom we have a small dense positively charged nucleus with the electrons simply spinning around it. Since the electrons have a negative charge and the nucleus had a positive charge what was there to stop the electrons simply crashing into the nucleus? The answer to this problem was provided by Niels Bohr and led to the idea of electron shells or energy levels in atoms.
Following Rutherford's work we have a small dense
nucleus surrounded by a dense cloud of electrons. Some scientists had the idea that the
internal structure of an atom could resemble that of the solar system where we
have planets orbiting the Sun; could a similar model exist for atoms? Where we
could have electrons orbiting the nucleus.
Niels Bohr, a Danish physicist suggested that the electrons orbit the
nucleus
in discrete well defined orbits at set distances from the nucleus. This is
similar to the solar system model but different in one crucial way. The
electrons are only allowed to orbit the
nucleus in certain shells or energy levels.
What this means in practice is that an electron could be in energy level 1 or
electron
shell 1 or in electron shell 2 but it CANNOT be an energy level somewhere between energy
level 1 and energy level 2. A physicist might say that the energy levels are quantized
meaning only certain values are allowed. The first electron shell is lowest in energy
and the second electron shell is higher in energy than shell 1 and so on..... electrons
occupy the lowest energy level or shell possible at any given time.
In 1932 James Chadwick discovered the neutron. His discovery followed work from a number of scientists who had noticed that when alpha particles struck certain materials an unusual type of radiation was emitted.
Polonium is a highly radioactive element which emits alpha particles. It had been noted that if certain elements e.g. beryllium where placed in the path of
alpha particles a strange unknown radiation was produced; see diagram below.
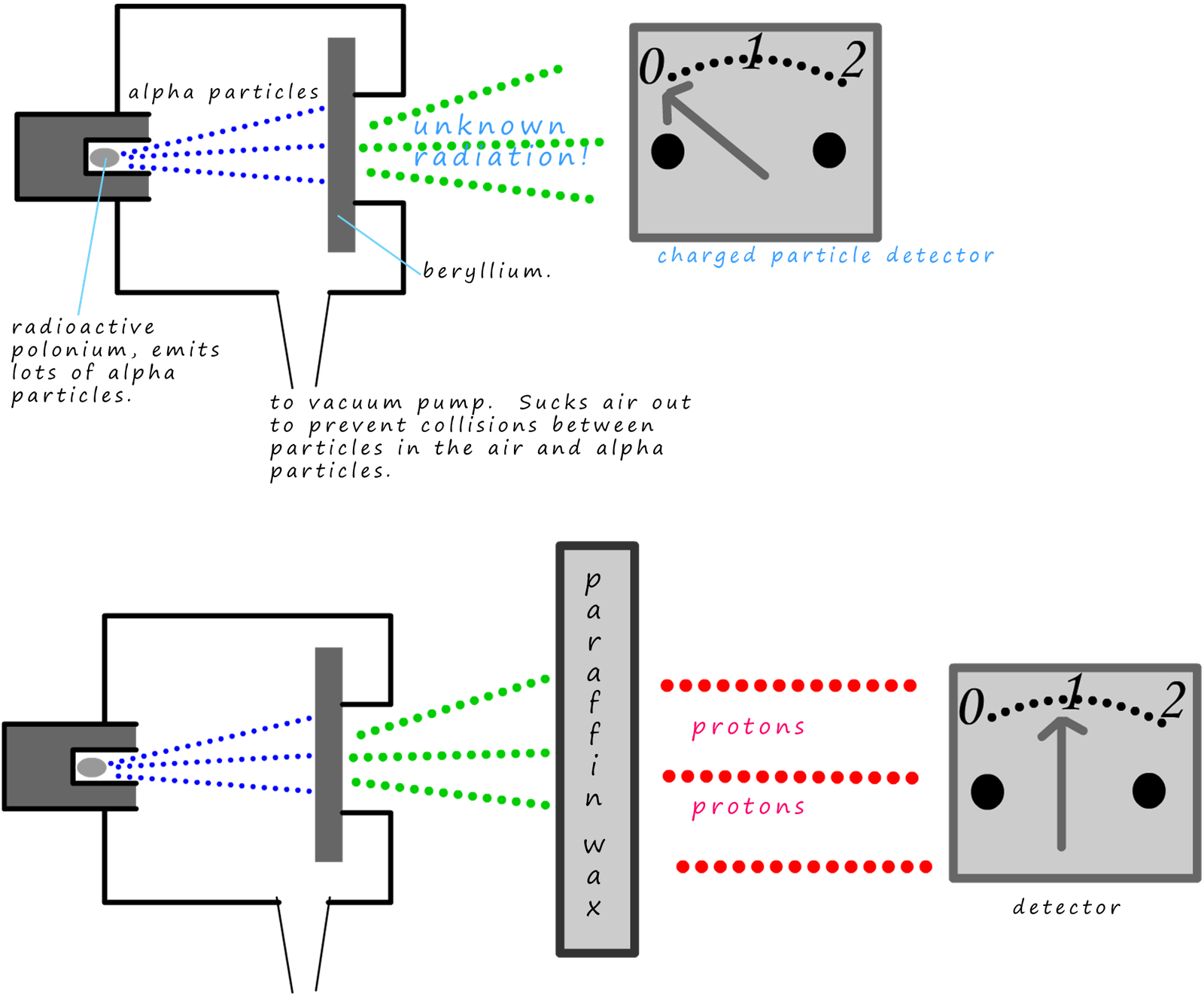 Chadwick realised that when the alpha particles from the polonium hit the
beryllium the unknown radiation which was produced was uncharged but deeply
penetrating. It was not picked up by the charged particle detector. Paraffin wax when
struck with this unknown new radiation emitted protons. The protons then are recorded by
the detector. Chadwick's measurements and calculations enable him to realise that this
unknown radiation was in fact neutrons.
Chadwick realised that when the alpha particles from the polonium hit the
beryllium the unknown radiation which was produced was uncharged but deeply
penetrating. It was not picked up by the charged particle detector. Paraffin wax when
struck with this unknown new radiation emitted protons. The protons then are recorded by
the detector. Chadwick's measurements and calculations enable him to realise that this
unknown radiation was in fact neutrons.
Summary of Rutherford's gold foil experiment: