

Higher and foundation tiers
 When we think of metals we usually think of a shiny, hard material which is strong and fairly
unreactive. However not all metals fit this description, some metals are soft and very reactive.
Group 1 in the periodic table is called the alkali metals; it contains the
metals lithium, sodium,
potassium, rubidium, caesium and francium. These metals are all very reactive and must be handled
with great care. Lithium at the top of group 1 is the least reactive alkali metal and as we go down the group these
metals react more and more violently.
When we think of metals we usually think of a shiny, hard material which is strong and fairly
unreactive. However not all metals fit this description, some metals are soft and very reactive.
Group 1 in the periodic table is called the alkali metals; it contains the
metals lithium, sodium,
potassium, rubidium, caesium and francium. These metals are all very reactive and must be handled
with great care. Lithium at the top of group 1 is the least reactive alkali metal and as we go down the group these
metals react more and more violently.
Since these metals are all in group 1 of the periodic table they
all have one electron in their
last shell, so losing this electron will give them a full outer
electron shell (stable electronic structure). Losing
their outer electron will mean they will form ions with a +1 charge e.g. Li+, Na+, K+,
Rb+, Cs+.

The physical properties of the alkali metals are also very different from what you might expect of a typical metal. The table below lists the melting and boiling points of the alkali metals as well as their densities.
| alkali metal | melting point/0C | boiling point/0C | density(g/cm3) at 250C |
|---|---|---|---|
| lithium | 180 | 1347 | 0.53 |
| sodium | 98 | 883 | 0.97 |
| potassium | 63.5 | 774 | 0.86 |
| rubidium | 39 | 696 | 1.53 |
| caesium | 28 | 669 | 1.87 |
To help explain how these properties of the alkali metals differ with those of some everyday metals compare the values in the table below with the table above.
| metal | melting point/0C | boiling point/0C | density(g/cm3) at 250C |
|---|---|---|---|
| aluminium | 660 | 2519 | 2.7 |
| tungsten | 3422 | 5555 | 19.3 |
| iron | 1535 | 2861 | 7.8 |
| titanium | 1668 | 3287 | 4.5 |
By studying the information in the table it is fairly clear that the alkali metals have unusually low melting and boiling points when compared to other metals, their densities are also low when compared to other metals. The density of water is 1 g/cm3, so any metal with a density less than this will float in water. So from the table above it is clear that the alkali metals lithium, sodium and potassium will all float on water since they have densities of less than 1g/cm3. However the alkali metals react violently with water, this is one of the reasons why they are stored in jars containing oil or paraffin, this ensures that water is kept well away from these reactive metals.
We can summarise the contents of the tables above as:
The alkali metals are all group 1 metals
so they will have 1 electron 1 their outer electron
energy
level. Since the atoms present in the alkali metals get larger as you descend the group, this outer negatively charged electron
is further away from the
attraction of the positively charged nucleus and so is more easily lost or removed, this is the same as saying that less energy will be needed to remove it.
This means that the reactivity of the alkali
metals will increase as you descend the group.
Having just one electron in their outer shell means that when they
react; the alkali metals will lose this
electron and form ions with a 1+ charge.
 The alkali metals react very violently
with water to form
alkaline
solutions and the flammable
gas hydrogen is also released. The image below shows the typical reactions of the first three alkali metals lithium, sodium and potassium
with water. Enough heat is generated in these reactions with the
water that the metal may actually melt and form a
ball of molten liquid metal that shoots across the surface of the
water.
The alkali metals react very violently
with water to form
alkaline
solutions and the flammable
gas hydrogen is also released. The image below shows the typical reactions of the first three alkali metals lithium, sodium and potassium
with water. Enough heat is generated in these reactions with the
water that the metal may actually melt and form a
ball of molten liquid metal that shoots across the surface of the
water.
The reaction of water with potassium is violent enough that the hydrogen gas released will ignite by itself and burn with a mauve flame (lilac or pale purple). Sodium and lithium react less violently and the hydrogen produced here will not ignite and burn by itself but will have to be lit by a burning splint, however once lit the gas will continue to burn on its own. Sodium colours the hydrogen flame yellow and lithium will turn the burning hydrogen gas brick red.
The image below illustrates the colours of the burning hydrogen flame above the three metals lithium, sodium and potassium. The alkali metals all react violently with water to form alkaline solutions (solutions of metal hydroxides). The reaction of potassium with water is so violent that the hydrogen gas released spontaneously catches fire to give a lilac coloured flame above the molten metal. Sodium and lithium also release hydrogen but this needs to be lit with a burning splint. The burning hydrogen gas above sodium is yellow and brick-red with lithium.

If a few drops of universal indicator are added to the water in the glass trough above then it will quickly turn purple once the alkali metals start reacting, showing that a strong alkali has been formed. The alkali metals rubidium and caesium being at the bottom of group 1 are even more reactive, they are denser than water and will sink but they react in a similar but much more violent way to Li, Na and K. All the alkali metals react with water in a similar way, they all form solutions of metal hydroxides (alkalis) and release the explosive gas hydrogen, this is outlined in the equations below:
Alkali metals get their name because they react with water to form alkaline solutions, lithium, sodium and potassium hydroxides are all strong alkaline solutions.
Similar violent reactions occur between the alkali metals and oxygen and chlorine gases to form oxides, peroxides, superoxides and chlorides, the same trends are always seen, the reactions become more violent the lower the metal is in group 1. The reason for this is that all alkali metals have 1 electron in their outer (last electron shell), therefore if they can lose this one electron they will end up with a stable or full last shell and as mentioned above less energy is required to remove this outer shell electron the further down group 1 the metal is found. Since the metals are losing 1 electron, the atom will end up with 1 more positively charged proton in the nucleus than negatively charged electrons in its shells, so it will end up forming a positive ion with a charge of +1.
The image below shows a possible set-up for the reaction the alkali metal sodium with chlorine gas. A small piece of sodium is placed on top of a mound of sand in the base of the flask. The flask is filled with dry chlorine gas. To start the reaction a single drop of water is dropped onto the sodium metal from the pipette, this immediately raises the temperature of the sodium metal; a very violent reaction now occurs between the sodium and chlorine; a bright flash is seen as the sodium and chlorine react. The flask fills up with white "smoke"; which is solid sodium chloride.
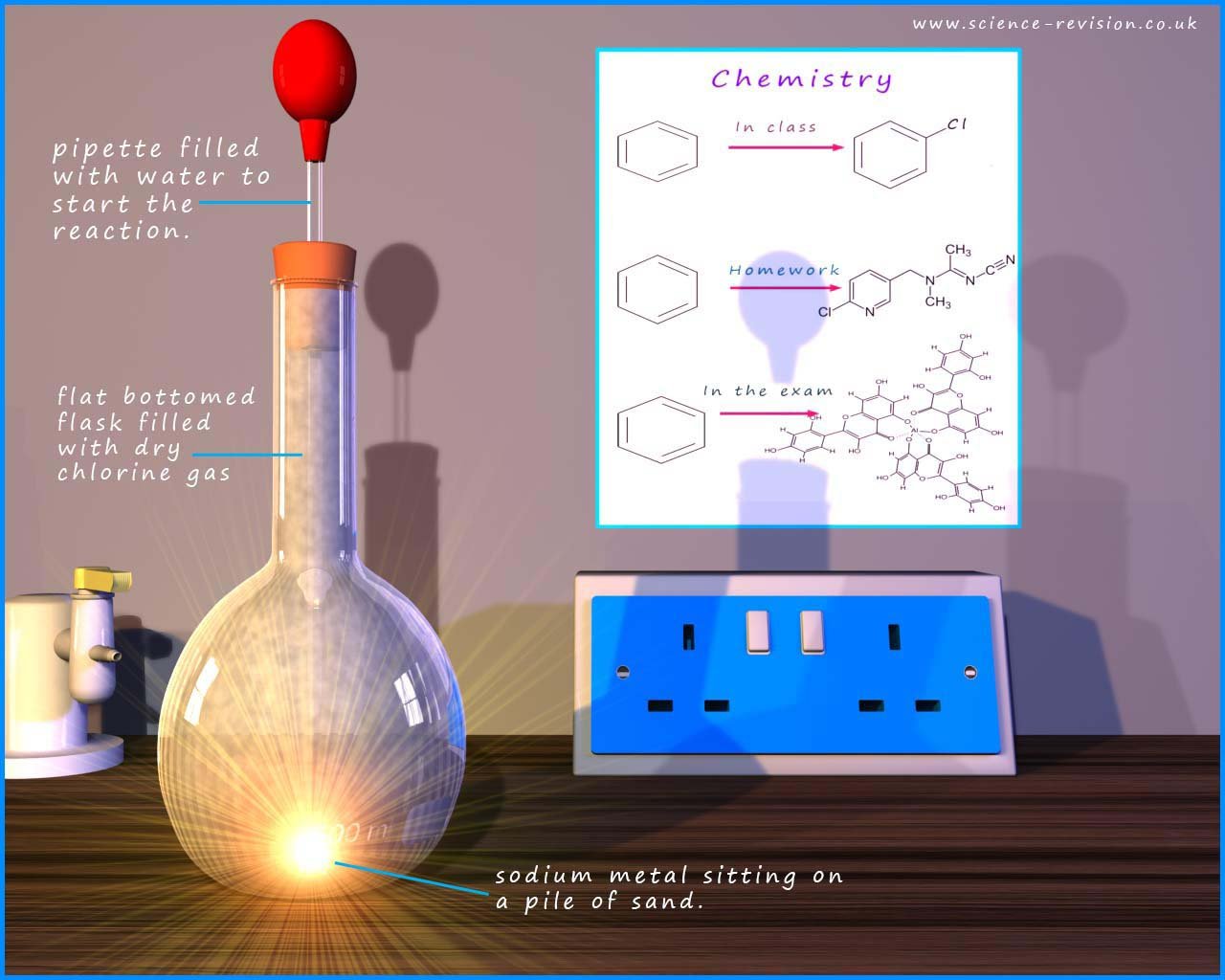
The alkali metals react with the halogens (F, Cl, Br, I) to form colourless ionic solids. The reactions
are very exothermic and can be violent. The reactions follow the expected trends, the more reactive
the alkali metal and the more reactive the halogen the
more violent and explosive the reaction. The reaction can be
summarised as:
So for example when sodium reacts with chlorine to form sodium chloride we have:
The products of the reaction of an alkali metal with oxygen depend on its reactivity. Normally when oxygen reacts it gains 2 electrons to form the oxide ion, O2-. This ion is particularly stable since the oxide ion has full octet or 8 electrons in its outer shell. However in the reactions of oxygen with the alkali metals other ions of oxygen are also formed. One of these ions, the peroxide ion has the formula O2 2- and it forms when sodium and potassium react with oxygen. Reactive alkali metals such as potassium, rubidium and caesium are also able to form an oxide called a superoxide. The superoxide ion has the formula O2-
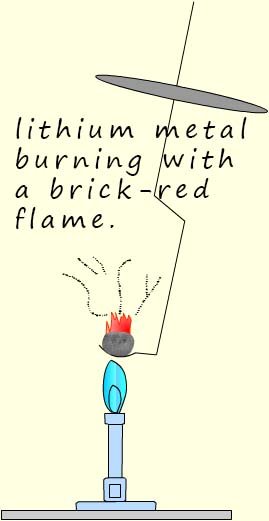 If a piece of lithium is placed on a burning spoon and heated strongly
in air it burns with a brick red flame
to form lithium oxide:
If a piece of lithium is placed on a burning spoon and heated strongly
in air it burns with a brick red flame
to form lithium oxide:
If a piece of sodium is placed on a burning spoon and heated strongly in air it burns with a yellow flame to form a mixture of two products, sodium oxide and sodium peroxide; with the sodium peroxide being the main product of this combustion reaction:
 Potassium is the most reactive alkali metal studied in GCSE chemistry.
The very reactive alkali metals from potassium onwards are able
to form an oxide called a superoxide. The formula for the
superoxide ion is O2-.
Potassium is the most reactive alkali metal studied in GCSE chemistry.
The very reactive alkali metals from potassium onwards are able
to form an oxide called a superoxide. The formula for the
superoxide ion is O2-.
So when a piece of potassium metal is burned in air a lilac coloured flame is seen and a mixture of two
oxides are produced; these are potassium peroxide and potassium superoxide as the main product of this combustion reaction.
When the two alkali metals caesium (Cs) and rubidium (Rb) burn in oxygen, they predominantly form superoxides but some metal peroxide may also form.
Use the flashcards below to review your understanding of the main properties and reactions of the alkali metals.









Complete the true/false activity below by simply placing the statements into either the true or false box. Press the check answer button when your done.
Drag statements into the bins or click a statement, then click a bin.
