

Higher and foundation tiers
 The group 7 non-metals are called the halogens.
The halogens are fluorine, chlorine, bromine and
iodine. Astatine at the bottom of group 7 is a very rare and highly radioactive element and it is very unlikely that you will ever come across it; its most stable isotope has a half-life of just over 8 hours.
The halogens are all very reactive elements and are not found as pure elements in nature; instead they are
found combined in various compounds in rocks and minerals.
Fluorine, chlorine and bromine are all toxic and corrosive elements and great care is needed when handling these reactive elements. Iodine being close to the bottom of group 7 is one the least reactive halogen and being a solid is the easiest and safest halogen to handle
in the lab.
The group 7 non-metals are called the halogens.
The halogens are fluorine, chlorine, bromine and
iodine. Astatine at the bottom of group 7 is a very rare and highly radioactive element and it is very unlikely that you will ever come across it; its most stable isotope has a half-life of just over 8 hours.
The halogens are all very reactive elements and are not found as pure elements in nature; instead they are
found combined in various compounds in rocks and minerals.
Fluorine, chlorine and bromine are all toxic and corrosive elements and great care is needed when handling these reactive elements. Iodine being close to the bottom of group 7 is one the least reactive halogen and being a solid is the easiest and safest halogen to handle
in the lab.

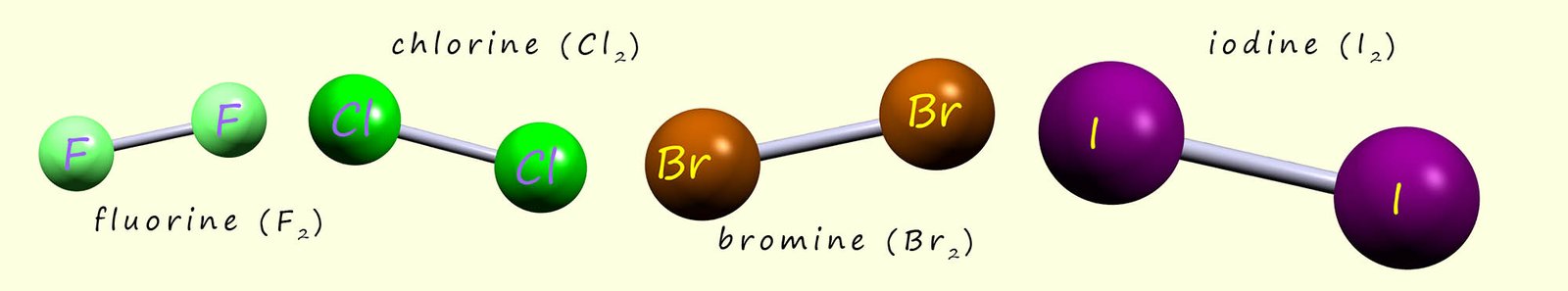
The halogens "go around in pairs"- that is they form molecules made up of two atoms as shown in the image. These diatomic molecules or two atom molecules are quite common for non-metal elements e.g. oxygen, nitrogen and hydrogen gases also form these diatomic molecules.
The table below lists the melting and boiling points of the halogens. The trend or pattern is fairly obvious, as we go down group 7 the halogen molecules get larger and the relative mass increases. Larger molecules contain more electrons and this will result in stronger intermolecular bonding and this along with the increase in relative mass results in higher melting and boiling points.
| Halogen | Colour | Melting point/0C | Boiling point/0C | state at room temperature |
|---|---|---|---|---|
| fluorine | pale yellow | -220 | -188 | gas |
| chlorine | greenish-yellow | -101 | -34 | gas |
| bromine | red-brown | -7 | 59 | liquid |
| iodine | greyish-purple | 114 | 131 | solid |

All the halogens have 7 electrons in their outer shell, so only need to gain one to achieve full last shells. This means that the halogens are used as oxidising agents. That is they will accept electrons from other elements, they oxidise them and by accepting electrons and they are themselves reduced e.g. all the halogens react with iron wool. The trends in these reactions are what you might expect:
 Iron is a fairly unreactive metal but still displays the trends you would expect. The strongly
oxidising fluorine immediately accepts an electron from the iron atoms in the iron wool. The products of the
reaction are:
Iron is a fairly unreactive metal but still displays the trends you would expect. The strongly
oxidising fluorine immediately accepts an electron from the iron atoms in the iron wool. The products of the
reaction are:
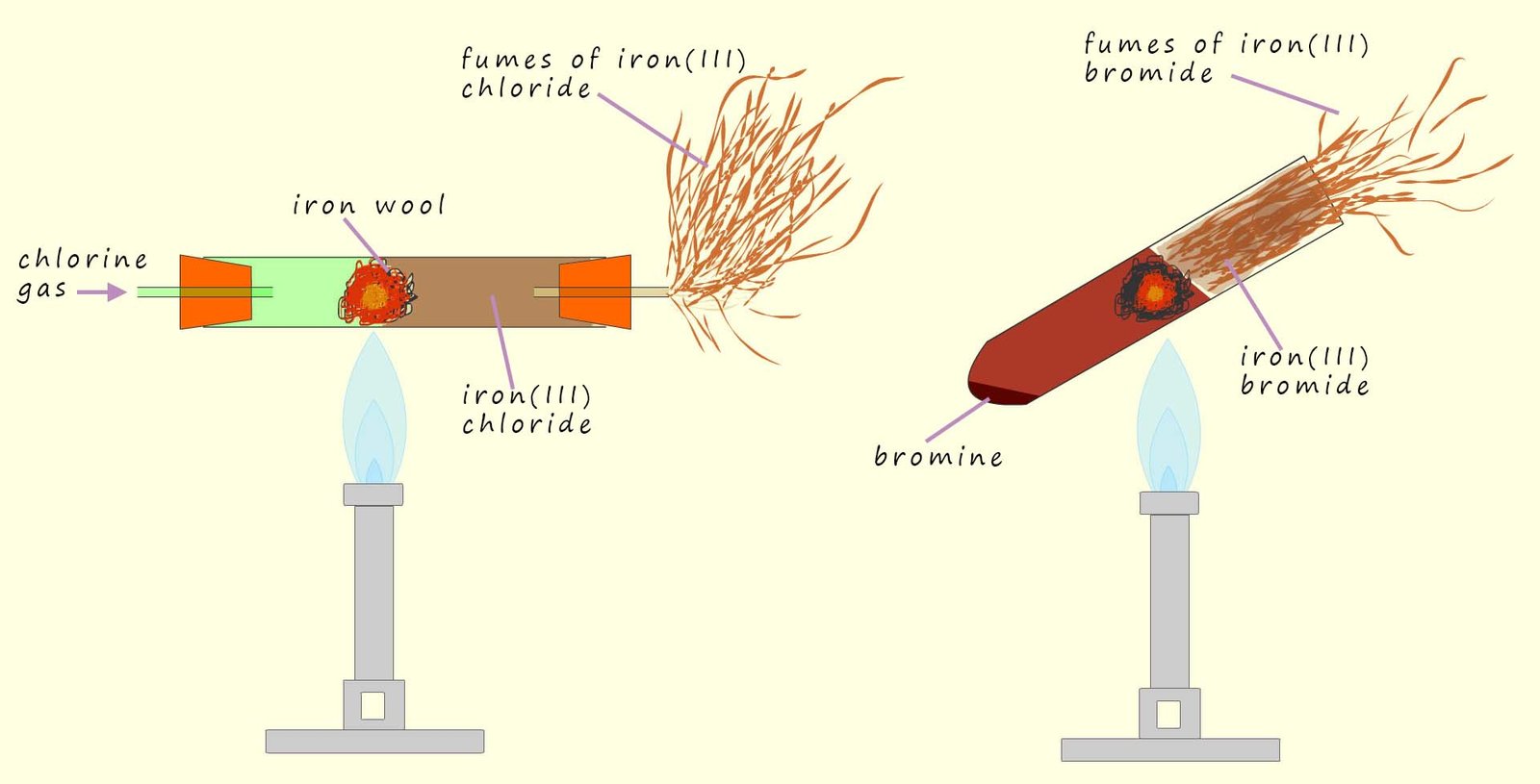
whereas the weakly oxidising iodine reacts much more slowly than chlorine or bromine and some considerable heat is needed to start the reaction. The other halogens all oxidise the iron to produce Fe3+ ions but the weakly oxidising iodine is only able to oxidise the iron to produce Fe2+ ions.
The metal iron in the above reactions can be replaced with more reactive metals and some spectacular reactions can be seen, for example the
halogens all react
with aluminium to give some very spectacular reactions. This should be predictable since aluminium is a much
more reactive metal than iron, so is more easily oxidised (remember oxidation = loss of electrons). The reaction
of aluminium metal with chlorine gas is shown in the image below.
 The equation for this reaction can be shown as:
The equation for this reaction can be shown as:
The reaction of iodine and aluminium is much slower than that of chlorine or bromine. In fact the two substances can be
mixed fairly safely on a clean dry tin lid. A few drops of water are added to this mixture to catalyse and start the reaction. After about 60 seconds or so the reaction starts.
A bright vivid glow is seen as the aluminium iodide forms, but perhaps the most vivid part of the reaction is
the dense violet coloured fumes of iodine vapour that are produced. The heat produced by the reaction
causes some of the iodine to sublime into
iodine vapour. This is outlined in the image below:
 In fact any reactive metal will react with the halogens to form salts called metal halides. The salts formed from these
reactions are all ionic compounds. The metal atoms lose electrons and are oxidised in these reactions to form positively charged metal ions. The halogens
always gain electrons; that is they are reduced to form negatively charged halide ions.
In fact any reactive metal will react with the halogens to form salts called metal halides. The salts formed from these
reactions are all ionic compounds. The metal atoms lose electrons and are oxidised in these reactions to form positively charged metal ions. The halogens
always gain electrons; that is they are reduced to form negatively charged halide ions.
Answer the three questions below to check your understanding of some of the key points from above. Click the boxes to reveal the answers.
 Fluorine is a pale greeny yellow gas while chlorine is a pale green coloured gas at room temperature. Bromine is a red-brown liquid and iodine is a
greyish/purple solid with a metallic like sheen.
Fluorine is a pale greeny yellow gas while chlorine is a pale green coloured gas at room temperature. Bromine is a red-brown liquid and iodine is a
greyish/purple solid with a metallic like sheen.
 The halogens are small molecules composed of only two atoms. Diatomic molecules are molecules composed of only two atoms. The formula of the first four halogens is F2, Cl2, Br2 and I2 clearly indicating that they are diatomic molecules.
The halogens are small molecules composed of only two atoms. Diatomic molecules are molecules composed of only two atoms. The formula of the first four halogens is F2, Cl2, Br2 and I2 clearly indicating that they are diatomic molecules.
 An oxidising agent is a substance that accepts electrons or removes electrons from another atom or molecule. The halogens all have 7 electrons in their outer electron shell and so are looking to gain one electron to achieve an octet of electrons or full last shell. When the halogens react with metals such as magnesium or aluminium they will remove electrons from these metals; that is they will oxidise the metals and act as oxidising agents.
An oxidising agent is a substance that accepts electrons or removes electrons from another atom or molecule. The halogens all have 7 electrons in their outer electron shell and so are looking to gain one electron to achieve an octet of electrons or full last shell. When the halogens react with metals such as magnesium or aluminium they will remove electrons from these metals; that is they will oxidise the metals and act as oxidising agents.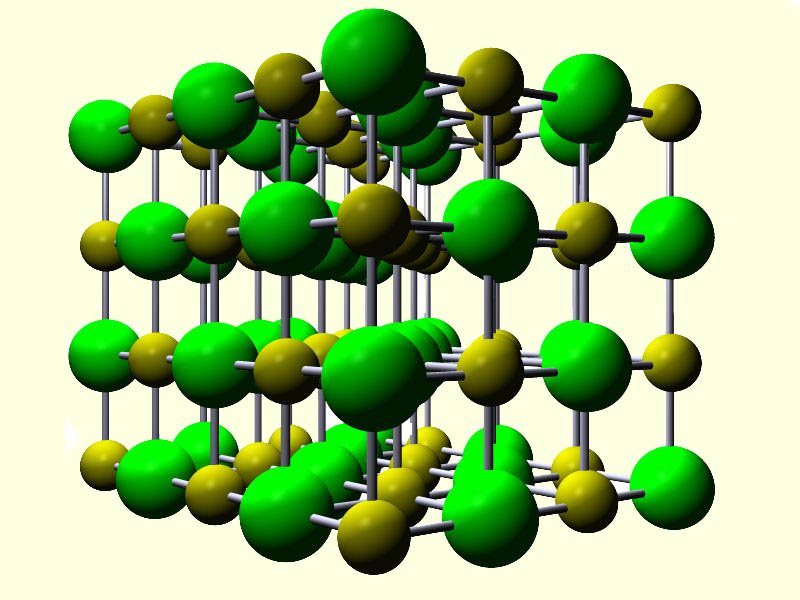
When the halogens react with a metal from group I or II in the periodic table the compound formed will be an ionic compound with a giant ionic lattice structure. These compounds will consist of negatively charged halide ions and positively charged metal ions. As an example consider the reaction of the alkali metal sodium with the halogen chlorine.
This is a very violent reaction and the product formed, sodium chloride has a giant ionic lattice (shown opposite) which consists of positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-).
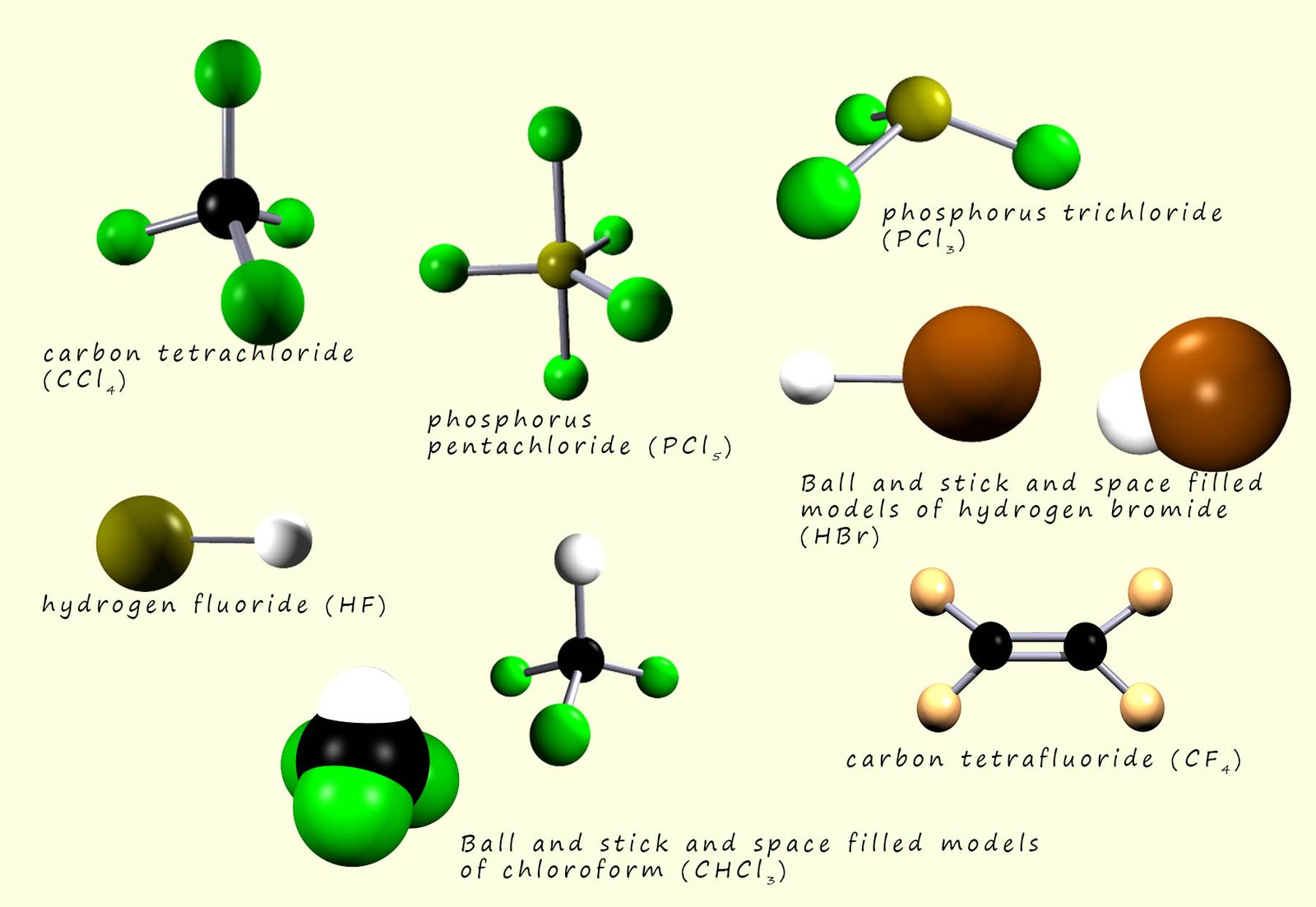
While the halogens react with metals to form compound which have a giant ionic lattice structure; however when the halogens react with other non-metal elements they form compounds with simple molecular structures. The image below shows a range of different halogen compounds which consist of only non-metal atoms. Here all the compounds have simple molecular structures.
 Perhaps one of the best reactions to show the reactivity trends in the halogens is their reaction
with hydrogen gas. All the halogens react with hydrogen to form hydrogen halide vapours:
Perhaps one of the best reactions to show the reactivity trends in the halogens is their reaction
with hydrogen gas. All the halogens react with hydrogen to form hydrogen halide vapours:
Fluorine being the smallest halogen atom will be able to attract a negatively charged electron from a metal atom more strongly towards its positively charged nucleus and so is the most reactive halogen. Iodine being in period 5 of the periodic table has 5 shells of electrons between its nucleus and any electron it tries to attract, these shells shield the positively charged nucleus from any electron that it is trying to attract. The iodine nucleus may have a much larger positive charge than the small fluorine nucleus, but the effect of shielding and the fact that the nucleus is a long way from any electrons it may try and attract means that the ability to attract electrons decreases as you descend group 7.