

Higher and foundation tiers
 Element number 17 on the periodic table is chlorine. Its chemical symbol is shown opposite now
chlorine has an atomic number of 17; this means it contains 17 protons in the
nucleus and since it is an atom it will be neutral with no overall charge and so it will also have 17 electrons. To calculate
the number of neutrons in a chlorine atom you simply subtract the atomic number from the
mass number; so we have
35.5-17 = 18.5 neutrons!! Obviously you cannot have half a
neutron so does this mean that the mass given in the periodic table is wrong?
Well no but it's a key clue that the mass shown on the periodic table isn't a simple count of protons and neutrons and we'll explore why this is the case shortly
Element number 17 on the periodic table is chlorine. Its chemical symbol is shown opposite now
chlorine has an atomic number of 17; this means it contains 17 protons in the
nucleus and since it is an atom it will be neutral with no overall charge and so it will also have 17 electrons. To calculate
the number of neutrons in a chlorine atom you simply subtract the atomic number from the
mass number; so we have
35.5-17 = 18.5 neutrons!! Obviously you cannot have half a
neutron so does this mean that the mass given in the periodic table is wrong?
Well no but it's a key clue that the mass shown on the periodic table isn't a simple count of protons and neutrons and we'll explore why this is the case shortly
Now recall that all the mass of an atom is due to the presence of the protons and neutrons found inside the nucleus, for example the element carbon has 6 protons and 6 neutrons inside its nucleus so the mass number for an atom of carbon will be 12, the mass number is simply the sum of the number of protons and neutrons added together.
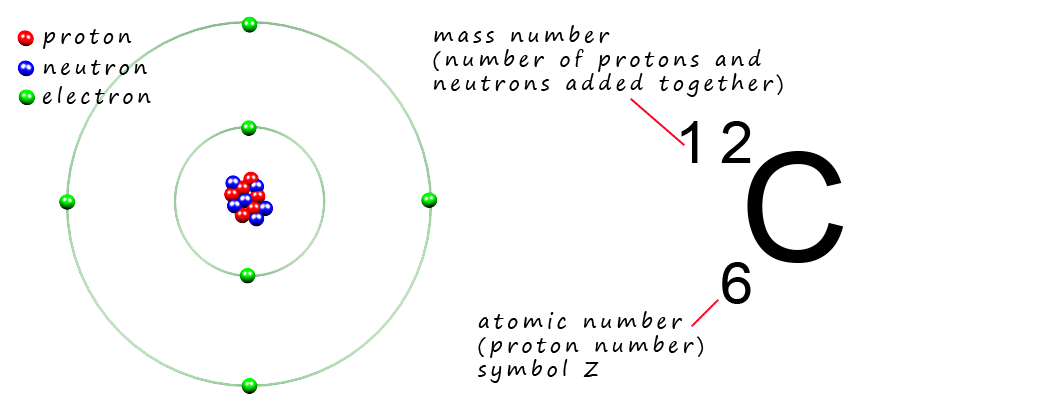
Now you are probably used to measuring masses in kilograms, but when we are dealing with something as small as atoms the kilogram is simply just too big a unit of measurement to use, after all you would not measure your mass in tonnes would you? It's just not an appropriate unit. You would measure the mass of a lorry in tonnes but not in grams, again grams is just too small a unit of mass to use.

The mass of a proton and a neutron are almost identical, they both have a mass of 1.67 x 10-27kg. This number is obviously very small and not exactly easy to handle or use. So instead of dealing with these very small awkward numbers scientists use a unit of mass called the atomic mass unit (amu or u) to measure the mass of atoms.
Using this scale the mass of a proton and a neutron are simply 1 amu or just 1u. So what exactly is an atomic mass unit, well its simply defined as 1/12thof the mass of an atom of carbon-12 (12C). Now the diagram above is an atomic structure diagram for a carbon atom containing 6 protons and 6 neutrons in its nucleus with 6 electrons in the electron shells, so imagine if you could weigh this carbon atom on a mass balance and get its mass in grams, then dividing by this mass by 12, then this mass would be the mass in grams of an atomic mass unit (amu or just u), the mass is 1.660538921 × 10-24. So if you divide the mass of a proton or a neutron in grams by the mass of 1 amu then you will find that the mass of a proton and a neutron are 1 amu.
It is this relative (relative just means compared to) mass scale which is used as a reference scale from which the mass of all the other atoms are measured from. Or we could simply say the masses of all other elements are measured by comparing them to an atom of carbon-12, which has a mass of exactly 12 amu or 12u. However if you look in detailed periodic table or Google the mass of the element carbon then will find that the mass of carbon as shown in the periodic table has a relative atomic mass of 12.011! This is a similar problem we found above for the element chlorine which has a relative atomic mass of 35.5, so at this point you might be wondering what is happening here! Well the reason that these relative masses are fractions is due to the presence of isotopes.
So before we look at isotopes let's look at an area that often causes confusion, mass numbers and relative atomic masses. The mass number of an atom is simply the sum of the number of protons and neutrons inside the nucleus. Mass numbers are not used in the periodic table; the periodic table shows the relative atomic mass of a particular element. This relative atomic mass is a weighted average mass taking into account the abundance of each isotope present; this is shown for the element chlorine in the image below. This might seem a little complicated to begin with but it is actually very straightforward to work out the relative atomic mass of an element; we will look look at how to calculate the relative atomic mass for the element chlorine below:
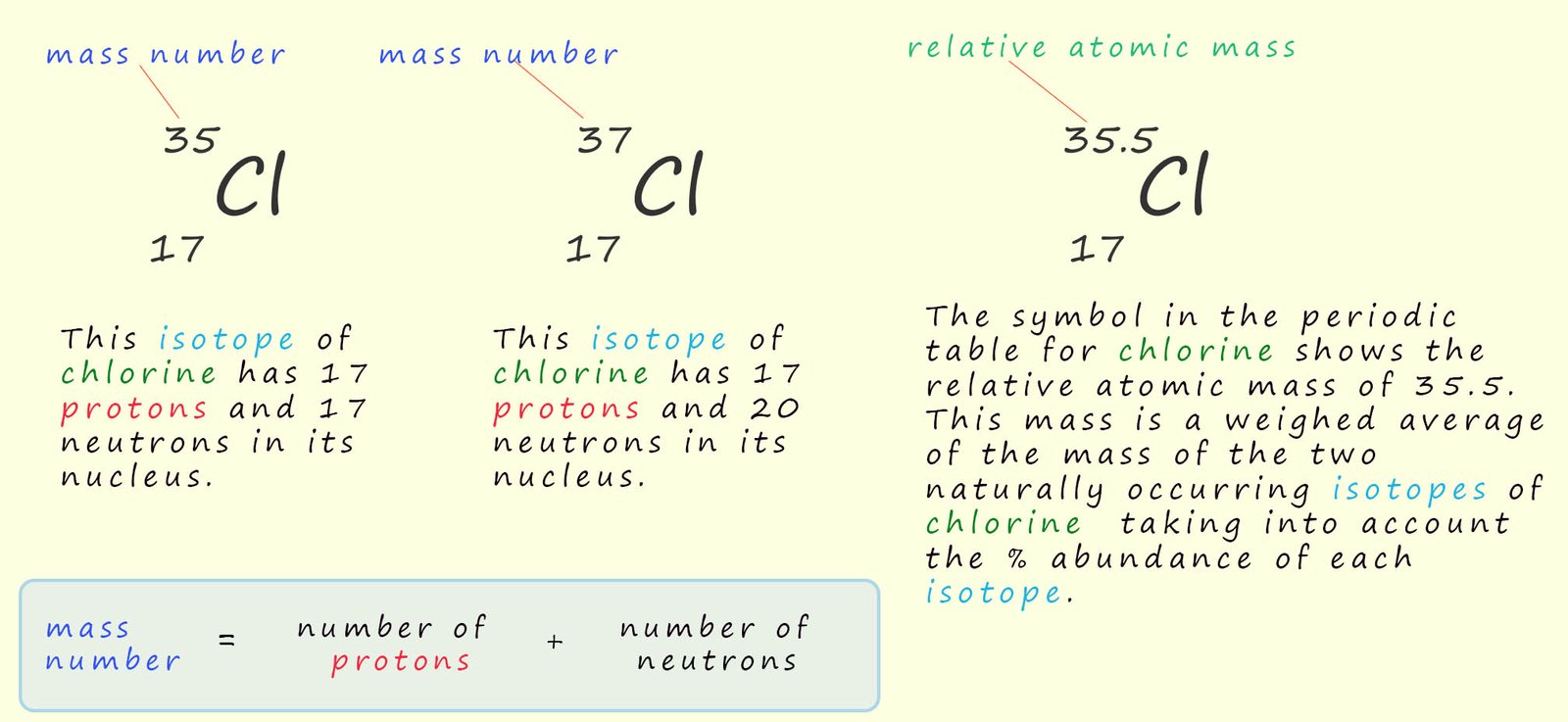
So the key points you need to be clear about are the masses given for a particular element in the periodic table are relative atomic masses and NOT mass numbers. The relative atomic mass is the weighted average mass for an element taking into account the presence of isotopes.
Now recall the following:

 All the elements in the periodic table have isotopes; some like chlorine have only two
isotopes while others have many more isotopes, for example caesium;
an alkali metal in group 1 of the periodic table has 40 isotopes! It might seem odd to think that the chlorine gas in the flask opposite contains different
types of chlorine atoms.
All of the chlorine atoms in the flask have 17 protons and 17 electrons
but some of them have extra neutrons and so will have a larger mass number.
All the elements in the periodic table have isotopes; some like chlorine have only two
isotopes while others have many more isotopes, for example caesium;
an alkali metal in group 1 of the periodic table has 40 isotopes! It might seem odd to think that the chlorine gas in the flask opposite contains different
types of chlorine atoms.
All of the chlorine atoms in the flask have 17 protons and 17 electrons
but some of them have extra neutrons and so will have a larger mass number.
Obviously you cannot use chemical tests to identify different isotopes of a particular element since these rely on the chemical reactions of the elements and these are determined by the electron arrangement of the atom and all isotopes have identical electron arrangements. However we can use differences in the physical properties of isotopes to identify them. For example some of the chlorine atoms have extra
neutrons and so they will have different masses,
boiling point is a physical property that varies with mass so in theory you could separate the different isotopes of chlorine using the fact that they will
have slightly different boiling points.
The fact that all elements have isotopes causes a bit of a dilemma- since each isotope has a different mass then what mass
do we record in the periodic table for an element?
As an example consider chlorine gas; now chlorine has two isotopes and these are shown below:

The two isotopes of chlorine have masses of 35 and 37 so which mass do we use for chlorine? Well we could take an average of the two masses and this would give an average mass of 36; (35 + 37)/2 = 36; however this average mass is not what is shown in the periodic table for chlorine. In the periodic table the mass of chlorine is given as 35.5. You may be wondering where the 35.5 has come from?
If each isotope of chlorine was present in equal amounts; that is 50% of chlorine atoms were 35Cl and 50% were 37Cl then we could simply take an average of the two masses which would give us an average mass of 36. However analysis of the two isotopes of chlorine shows that they are not present in equal amounts. 75% of all chlorine atoms are 35Cl while only 25% are 37Cl, so when working out the average mass we need to take into account the abundance of each isotope. The calculation you need to carry out is shown below:

The calculation gives a mass of 35.5; this is the relative atomic mass which is displayed in the periodic table for chlorine. The periodic table displays the relative atomic masses taking into account the abundance of each isotope.
As a final example consider the element hydrogen. Hydrogen has 3 naturally occurring isotopes. These are shown below.
| The three stable isotopes of hydrogen | ||
|---|---|---|
 |
 |
 |
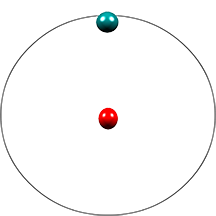
| This isotope called protium contains 1 proton in its nucleus. Its relative atomic mass is 1. It has 1 electron in the 1st electron shell. | This isotope called deuterium contains 1 proton and 1 neutron in its nucleus Its relative atomic mass is 2. It has 1 electron in the 1st electron shell | This isotope called tritium contains 1 proton and 2 neutrons in its nucleus. Its relative atomic mass is 3. It has 1 electron in the 1st electron shell |
As with all isotopes their chemical properties are identical. These 3 isotopes of hydrogen are no exception and all have identical chemical properties. The heavy isotopes deuterium (2H) and tritium (3H) are rare atoms with over 99% of all hydrogen atoms being protium (1H).
Test your understanding of isotopes by completing the paragraph below. Simply click the word or words in yellow then click the blue box in the paragraph where the selected word is to be placed.
Use the flashcards below to test your understanding of the key ideas mentioned above.