
Higher and foundation tiers
Imagine having to learn how all the elements in the periodic table react! That would probably be impossible. Luckily you don't have to. Once you know a little about the internal structure of atoms and in particular how the electrons are arranged within atoms; then you will have an excellent idea how each of the elements will react.
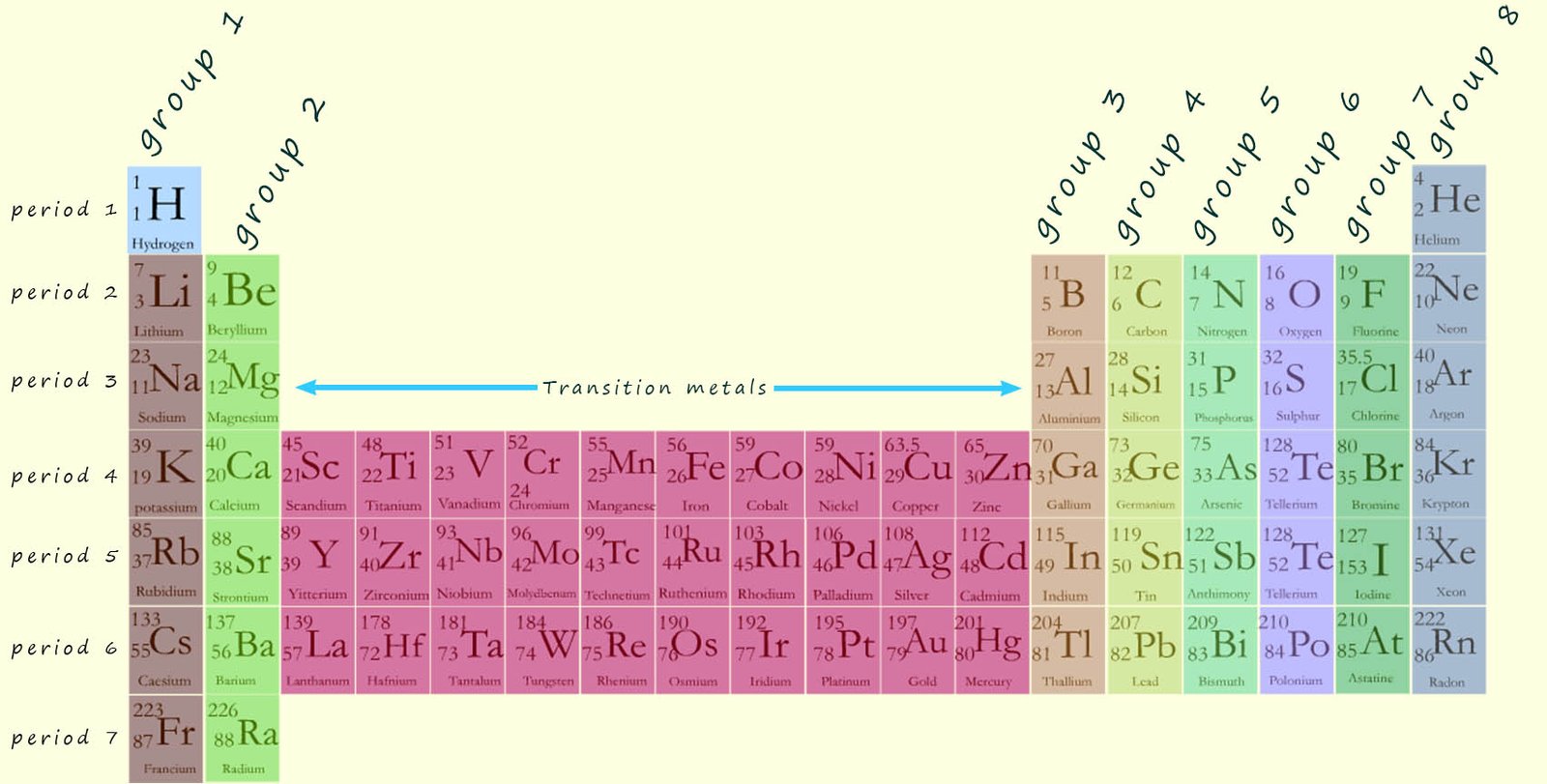
In the periodic table the elements are arranged in groups or columns. There are 8 groups; these are labelled in the image above as groups 1-7 with group 0 or 8, the Noble gases being the last group or column in the periodic table. In each group in the periodic table all the elements present in that particular group will all react in a similar way; so if you know how one element in any one group reacts then you can easily predict how the other elements in that particular group will react e.g. lithium and sodium are the two metals found at the top of group 1; the alkali metals.
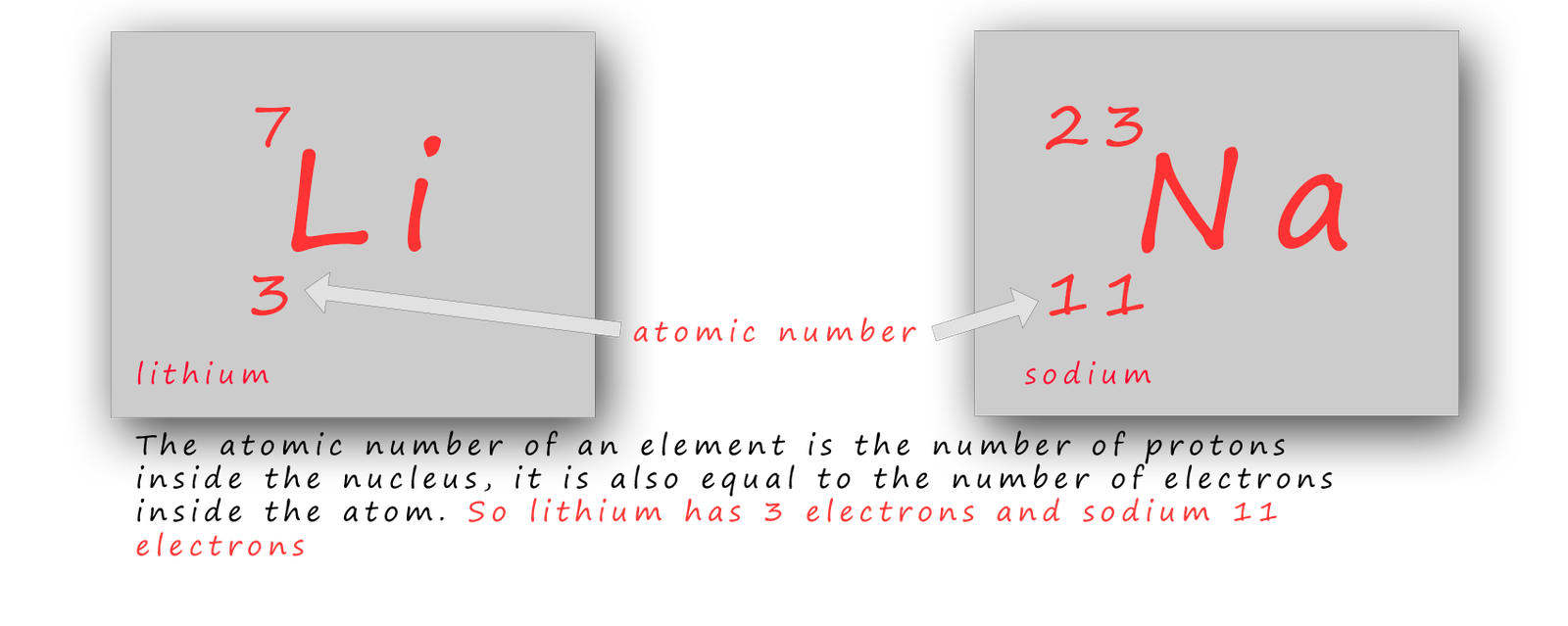
You have probably watched your teacher drop these two metals into a trough of water and seen the violent reaction that happens. Have you ever wondered why these two metals react in a similar way? Well the way an element reacts depends on how the electrons are arranged inside the atoms and in particular how many electrons are in the last or outer electron shell; which is often referred to as the valency shell.

The electrons fill up the electron shells in order. The first electron shell is the lowest in energy and it can hold a maximum of 2 electrons. Once the first electron shell is full then any additional electrons must go into the second shell. The second shell can hold a maximum of 8 electrons. Once this shell is full then any extra electrons go into the third shell, this shell also holds a maximum of 18 electrons or can it???
In many gcse courses the electron arrangements of atoms is often simplified and the 3rd shell is stated as only being able to hold a maximum of 8 electrons. While this simplification is generally not particularly helpful; many gcse course use it and since this site is based on the gcse course I will use this simplification in working out the electron arrangements for atoms.
You should be aware that in your gcse course you will not be asked to work out electron arrangements for any atoms above element 20; calcium. This is because this simplification on the number of electrons breaks down after element 20; calcium. Element 21 in the periodic table is scandium; which is the first of the transition metals and you will not have to work out the electron arrangements for any of the transition metals until you get to A-level chemistry.
 In your gcse course you will only need to be able to draw atomic structure diagrams similar to the one opposite for
any of the first 20 elements in the periodic table and we will assume the 3rd electron shell can only hold a maximum of 8 electrons but be aware that in reality it can actually hold up to 18 electrons!!
In your gcse course you will only need to be able to draw atomic structure diagrams similar to the one opposite for
any of the first 20 elements in the periodic table and we will assume the 3rd electron shell can only hold a maximum of 8 electrons but be aware that in reality it can actually hold up to 18 electrons!!
I have drawn out a few atomic structure diagrams below for a few
elements
but I would suggest you draw them out for the first 20 elements
in the periodic table and try to spot any patterns between the number of electrons in the last shell
and the group the element is found in the
periodic table.
Remember:
Let's go back to the examples above using lithium and sodium metal, both from group 1 in the periodic table. The table below shows how to work out the electron arrangement for these two alkali metals:
| Lithium has an atomic number of 3, this means it has 3 protons in its nucleus. It also has the same number of electrons as protons. To fit 3 electrons into the shells is fairly simple. .....2 electrons go into the first shell. .....1 electron then goes into the second shell. This means lithium has an electron arrangement or electron configuration of 2,1 |  |
Sodium has an atomic number of 11, this means it has 11 protons in its nucleus. It also has 11 electrons. To fit 11 electrons into the shells we do exactly as we did for lithium: .....2 electrons go into the first shell. .....8 electrons go into the second shell. ..... 1 electron goes into the third shell This means sodium has an electron arrangement or electron configuration of 2,8,1 | 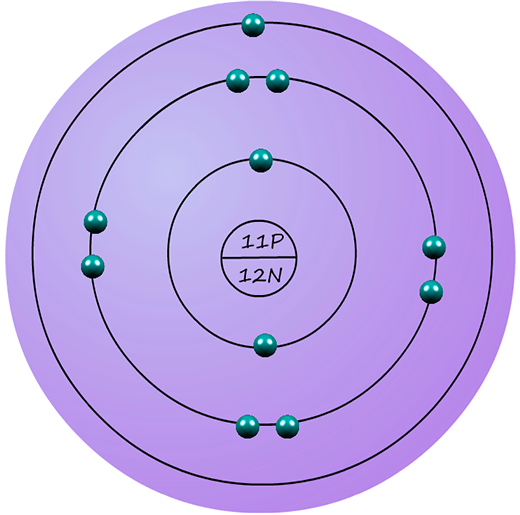 |

You should notice that both lithium (2,1) and sodium (2,8,1) have 1 electron in their outer electron shell. It is the number of
electrons in the outer shell
that determines how a particular element will react chemically with other substances. When you move down a row or period in the periodic table from lithium (2,1) to sodium (2,8,1)
one extra electron shell is added for each row or period you go down.
So although lithium (2,1) and sodium (2,8,1) do not have identical
electron arrangements, however they both have 1 electron in their outer shell and this
is why they react in a similar way. Potassium is the next element under sodium in group 1 of the periodic table.
Potassium has an atomic number of 19, so it will have 19 protons in its nucleus and 19 electrons in the electron shells. Since potassium is in group 1
of the periodic table it will also have 1 electron in
its outer electron shell.
Its electron arrangement will be 2,8,8,1. Again moving down one row or period from sodium to potassium in
the periodic table simply adds one extra electron shell.
There is an easy way to check if you have worked out the correct electron arrangement for an element; simply check what group in the periodic table the element is in and that particular element should have the same number of electrons in the last shell as the group it is in. The horizontal rows in the periodic table are called periods. Hydrogen and helium are in period 1, lithium is the first element in period 2 and sodium the first element in period 3. Whatever period an element is in will tell you how many electron shells it has e.g. study the information in table below and the periodic table at the top of the page and you should notice that the number of electron shells an element has is the same as the period in which the element is found in the periodic table.
| Element name | Group in periodic table | Atomic number | electron arrangement | Atomic structure diagram | period where element is found in the periodic table |
|---|---|---|---|---|---|
| carbon | 4 | 6 | 2,4 | 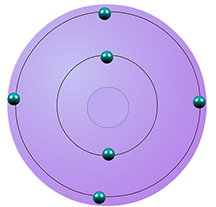 |
period 2 |
| silicon | 4 | 14 | 2,8,4 | period 3 | |
| nitrogen | 5 | 7 | 2,5 |  |
period 2 |
| phosphorus | 5 | 15 | 2,8,5 | 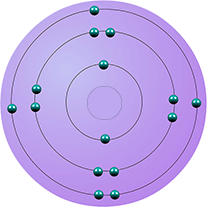 |
period 3 |
| oxygen | 6 | 8 | 2,6 | 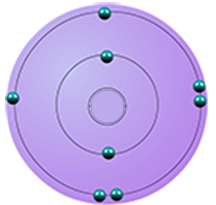 |
period 2 |
| sulfur | 6 | 16 | 2,8,6 | 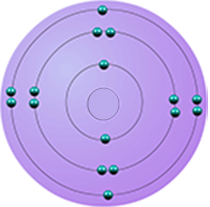 |
period 3 |
If you look at the elements in the table above you will notice that the group number where the element is found in the periodic table corresponds to the number of electrons in the last shell and the number of shells corresponds to the row or period the element is found in. This is the reason why chemists do not have to learn how every element reacts. Since chemical reactions depend upon the number of electrons in the last shell, then if you know how one element in any one particular group reacts with other substances then you can easily predict how all the other element in that group will react.