

Higher and foundation tiers
The non-metals are found on the left-hand side of the periodic table in groups 3, 4, 5, 6, 7 and group 0 (or 8). Group 7 is called the halogens and group 8 (or 0) is called the Noble gases or inert gases. The image shows an outline of where the non-metals are found in the periodic table.
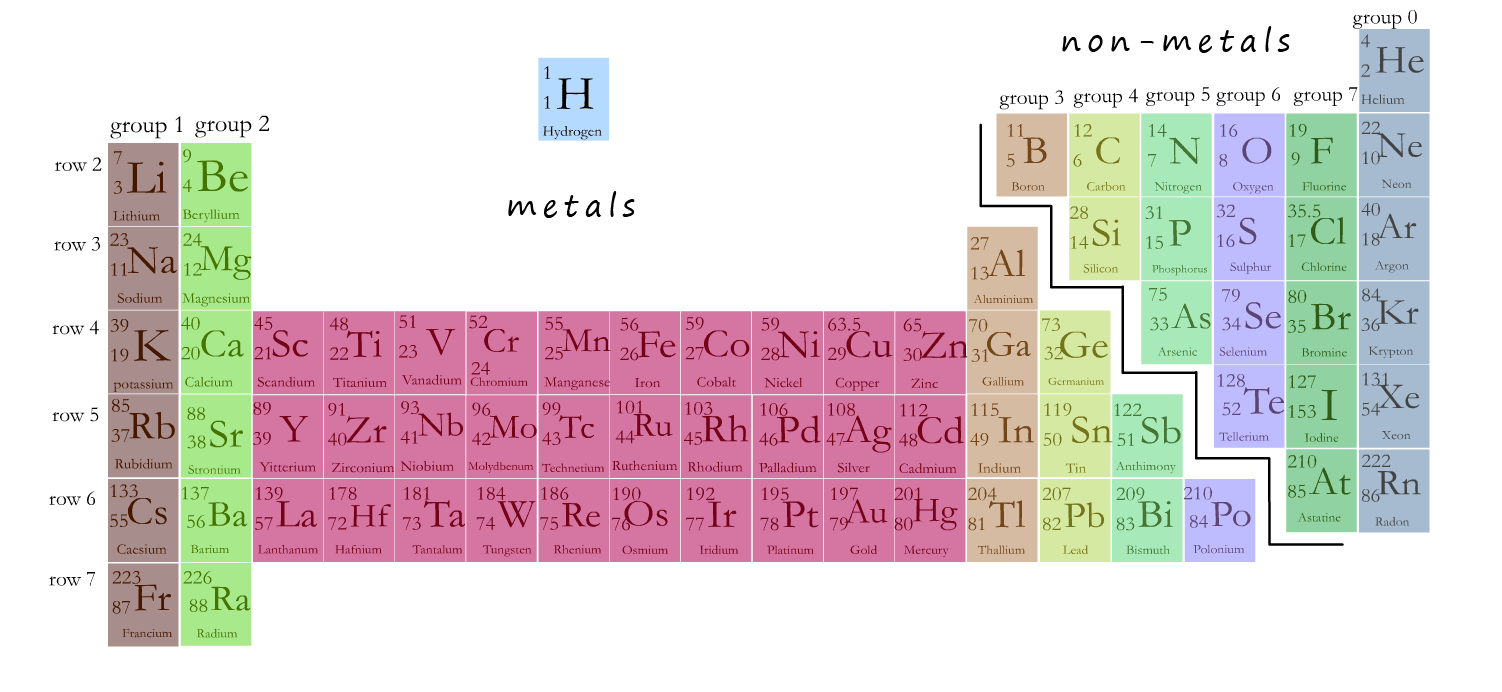
The physical properties of an element will depend largely on the type of structure it has and on the bonding present. The structure of the non-metal elements varies from the giant covalent network structures found in the group 4 elements such as carbon and silicon, however most of the non-metals have small molecular structures; for example the halogens; that is fluorine, chlorine, bromine and iodine are non-metal elements found in group 7 of the periodic table and they all have structures that consist of small diatomic molecules; that is molecules made up of just 2 atoms; as shown below:
The Noble gases are found in group 0 of the periodic table and these unreactive gases have structures that consist of individual single atoms, they have a monatomic structure.
Some of the physical properties of the non-metals elements are shown in the table below, where they are compared with those of metals.
| physical property of a typical non-metal | physical property of a metal |
|---|---|
| electrical insulator | electrical conductor |
| thermal insulator | thermal conductor |
| brittle (if solid) | malleable |
| low density | high density |
| low melting point | high melting point |
| dull (if solid) | shiny |
The image below shows the type of structure for most of the non-metals you are likely to meet. You can see from the image that the group 4 non-metals carbon and silicon have covalent giant structures but that groups 5, 6 and 7 non-metals have molecular structures. The Noble gases being almost inert consist of single atoms and are said to be monatomic gases.

 The group 5 elements nitrogen and phosphorus are the two elements you are most likely to meet.
These two elements have small
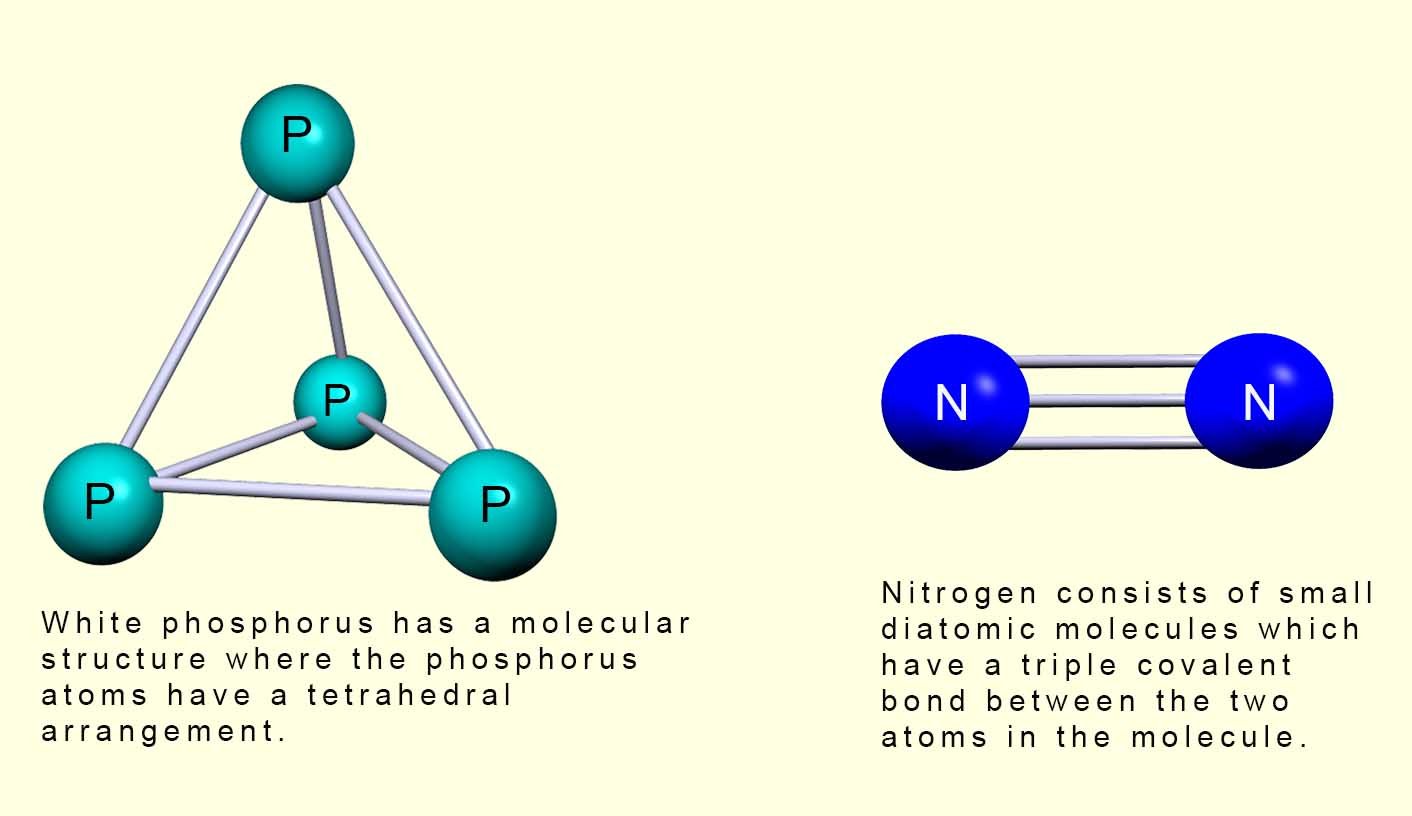
molecular structures. Nitrogen consists of two atoms joined together by a triple covalent bond
in a small diatomic molecule with the molecular formula N2. There are two common allotropes (types or forms)
of the element phosphorus, these are white phosphorus which
consists of a small molecule made of 4 phosphorus atoms joined in a tetrahedral shaped molecule,
which is shown in the image above. The other allotrope is red phosphorus which is a polymer made by linking lots of P4
tetrahedrons together to form a long chain.
The group 5 elements nitrogen and phosphorus are the two elements you are most likely to meet.
These two elements have small
molecular structures. Nitrogen consists of two atoms joined together by a triple covalent bond
in a small diatomic molecule with the molecular formula N2. There are two common allotropes (types or forms)
of the element phosphorus, these are white phosphorus which
consists of a small molecule made of 4 phosphorus atoms joined in a tetrahedral shaped molecule,
which is shown in the image above. The other allotrope is red phosphorus which is a polymer made by linking lots of P4
tetrahedrons together to form a long chain.
White phosphorus is a highly reactive element which can burst
into flames when exposed to oxygen in the air; this is why it is
stored under water. It is a
very dangerous element to handle and is used by the military in bombs and grenades and is one
of the main ingredients in incendiary weapons and smoke bombs.
These two elements have small molecular structures which consist entirely of covalent bonds, these small molecular structures also have weak intermolecular bonds between different molecules which means they have low melting and boiling points. White phosphorus has a melting point (m.p.) of 440C and its boiling point (b.p.) is 2800C. Nitrogen has a melting point of -2100C and its boiling point is -1950C. The image below shows the molecular structure of white phosphorus and nitrogen gas.
 The structure of two common group 6 elements sulfur and oxygen
are shown opposite. Sulfur is a very
common element and there are large underground deposits of elemental sulfur.
Sulfur is one of the few elements
that can be found relatively pure in the Earth's crust. It is a yellow solid with a
molecular structure.
There are
many different allotropes
(forms or types) of sulfur and many of them have
sulfur atoms in rings. Some of these
ring structures
contain 6 sulfur
atoms while other forms consist of ring structures containing up to 20 sulfur atoms. The most stable form at room temperature is
the allotrope containing 8 sulfur atoms; formula S8. This allotrope has a ring structure, this is shown in the image opposite.
The structure of two common group 6 elements sulfur and oxygen
are shown opposite. Sulfur is a very
common element and there are large underground deposits of elemental sulfur.
Sulfur is one of the few elements
that can be found relatively pure in the Earth's crust. It is a yellow solid with a
molecular structure.
There are
many different allotropes
(forms or types) of sulfur and many of them have
sulfur atoms in rings. Some of these
ring structures
contain 6 sulfur
atoms while other forms consist of ring structures containing up to 20 sulfur atoms. The most stable form at room temperature is
the allotrope containing 8 sulfur atoms; formula S8. This allotrope has a ring structure, this is shown in the image opposite.
Perhaps the most memorable fact about sulfur that you are likely to remember from using it
in the lab is the really bad smell many sulfur compounds have. Burning
sulfur produces sulfur dioxide; an
irritating foul smelling gas which smells of rotten eggs. Hydrogen sulphide gas (H2S) forms when sulfur
and hydrogen atoms join; this obnoxious smelling gas is used in stink bombs!!
Oxygen is a small diatomic molecule
containing 2 atoms held in place by a double covalent bond between the two atoms.
Like the elements in group 5 both sulfur and oxygen
have a small molecular structure with low melting
and boiling points. Sulfur melts at 1150C and boils at 4440C
while oxygen a much smaller molecule has a m.p. of -2190C and a b.p. of -1830C. Like all substances with covalent bonds these non-metals are
electrical insulators. The
reason for this is simply because
all the electrons in the outer shell are held in covalent bonds and are not free to move as they are in metals
Group 7 non-metals are called the halogens. The halogens are fluorine, chlorine, bromine and iodine. Astatine at the bottom of group 7 is a very rare and highly radioactive element. It has some uses in cancer treatments and other medical treatments.

The word halogen comes from
the Greek words meaning salt-former, hals (salt) and gennan meaning to form.
The halogens are
all very reactive elements and are not found as elements in nature, instead they are found
combined in compounds found in rocks and minerals.
They are all toxic and corrosive
and great care is needed in handling these reactive elements,
though iodine as the least reactive halogen
and being a solid is the easiest and safest halogen
to handle in the lab.
The halogens all consist of small diatomic molecules which means they will have low melting and
boiling points. The reactions and properties of the halogens are discussed in more detail on a separate page-
The halogens.
Fluorine and chlorine are coloured
gases at room temperature. Fluorine is a
green-yellowish gas similar in colour to chlorine but paler.
Bromine is a reddish brown volatile
liquid which produces very corrosive and toxic vapours. Iodine
is a purple coloured solid with a
reflective sheen. It sublimes with mild heating to form a beautiful violet coloured vapour.
Use the flashcards below to test your understanding of the main properties of the non-metal elements.
