
Higher and foundation tiers
 One of the many fun reactions you can carry out with halogens are displacement reactions.
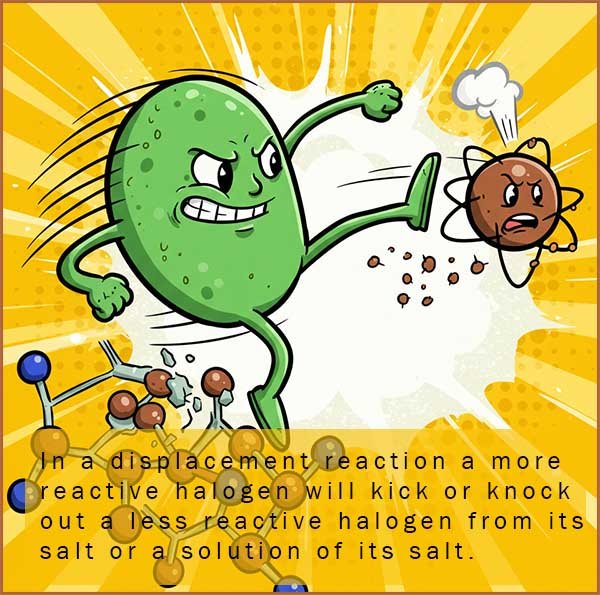
Displace means to kick-out or get rid of! Displacement reactions
are a good way to demonstrate
the order of reactivity of the halogens. Remember the most reactive
halogen is fluorine and the
reactivity of the halogens decreases as you descend group 7 of the periodic table.
One of the many fun reactions you can carry out with halogens are displacement reactions.
Displace means to kick-out or get rid of! Displacement reactions
are a good way to demonstrate
the order of reactivity of the halogens. Remember the most reactive
halogen is fluorine and the
reactivity of the halogens decreases as you descend group 7 of the periodic table.
In a halogen displacement reaction all that happens is a more reactive halogen will kick, remove out or displace a less reactive halogen from its solution or salt; for example if you have a solution of say potassium bromide; which contains the bromide (Br-) halide ion then if you add a more reactive halogen such as chlorine then it will knock out or displace the bromide ion from the solution and take its place to form a solution of potassium chloride and bromine, but more about these reactions later!
The halogens Chlorine, Bromine and iodine are all toxic gases, liquids and solids so they are often dissolved in water to make it easier and safer to handle them; for example chlorine dissolves in water to form a very pale green solution called chlorine water, similarly bromine dissolves to form a brown solution called bromine water. Iodine however is barely soluble in water; so to prepare iodine solution solid iodine is dissolved in a solution of potassium iodide and gently heated until the solid iodine dissolves to form a dark brown solution, which is simply referred to as iodine solution. Fluorine is simply too reactive to dissolve in water.

Fluorine will react with water to form a weak acid called hydrofluoric acid and a mixture of oxygen and ozone gases. It is highly unlikely that you will ever handle fluorine gas, fluorine is exceptionally dangerous and will react with almost everything; often violently. Fluorine gas is highly toxic gas; inhalation can cause severe respiratory damage which can be fatal and contact with the skin will cause deep and penetrating burns.
Recall also that a solvent is a liquid that is good at dissolving substances e.g. acetone is an
excellent solvent
used in nail varnish remover. Ethanol is another good solvent used in many perfumes and perhaps the
most common
solvent we use in science is simply water.
Some substances are soluble in water but many are not; for example if you add oil to
water it simply
floats on top of the water. Oil and water do not
mix, we would say that they are immiscible.
Many of the common solvents we use in
chemistry do not mix with water, but like oil they simply float on top of the
water; for example cyclohexane and hexane are two solvents which are immiscible with water and being less dense than water will simply float or sit on top of the water if the two liquids are mixed and then allowed time to separate out in a test tube or beaker.

Now as mentioned above the halogens chlorine, bromine and iodine are not that soluble in water and iodine is particular has a low solubility in water. Now since the halogens are toxic substances and are therefore dangerous to handle
they are often used in very dilute aqueous solutions, the problem with this is that colour changes are often used to identify if for example a halogen displacement reaction has taken place or not and these dilute solutions have very pale and washed out colours which can make it difficult to decide if a reaction has taken place or not.
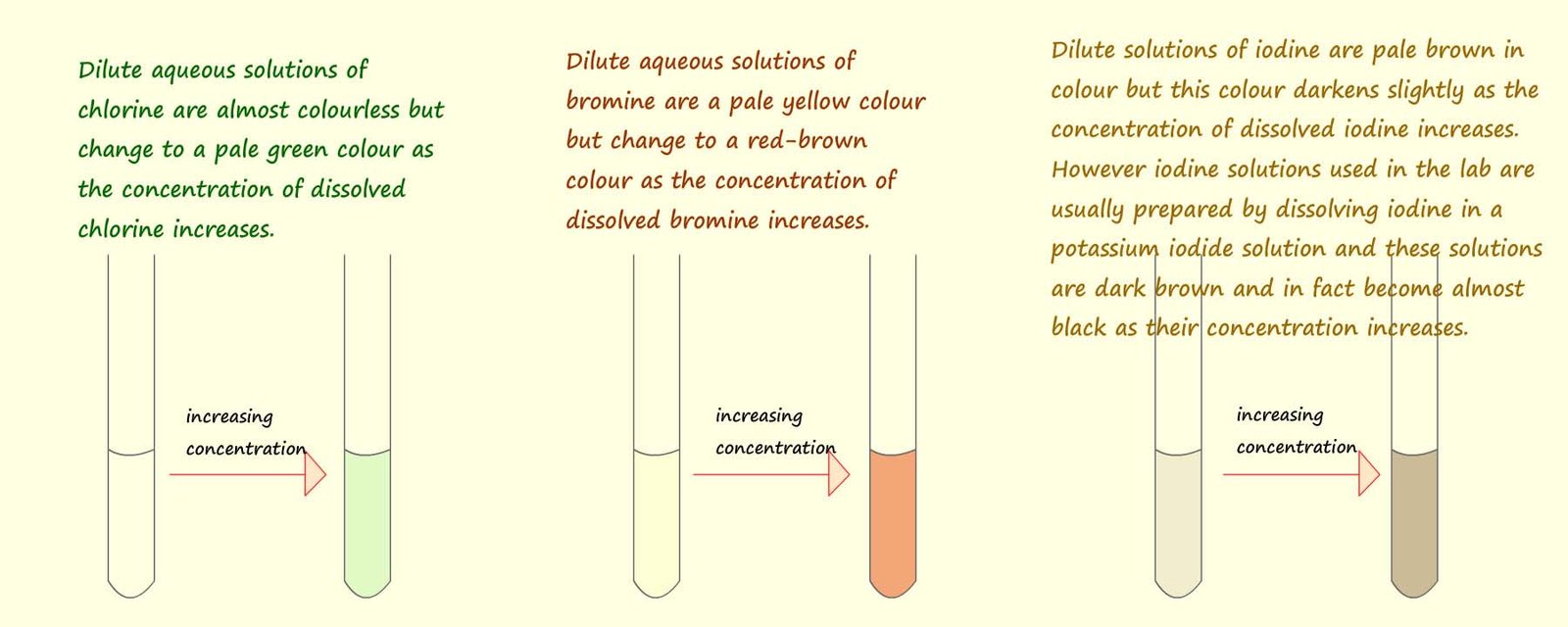
The image below shows how the colours of aqueous solutions of the halogens change as the concentration of the dissolved halogen changes. Though it is safer to handle dilute solutions of the halogens the pale and washed out colours make it a necessity to use the solvent cyclohexane or hexane to help identify any colour changes that take place during halogen displacement reactions.
The set-up for a typical halogen displacement reaction experiment is shown in the image below. In the first test-tube we have a solution of sodium iodide dissolved in water, this solution contains sodium ions (Na+) and bromide ions (Br-). On top of this is added a few centimetres of the solvent cyclohexane. Cyclohexane is a very good solvent for halogens and given the choice between dissolving in water and dissolving in cyclohexane, a halogen such as chlorine, bromine or iodine will always dissolve in cyclohexane before water. Cyclohexane is also immiscible with water; that is like oil it floats on top of the water. You can see this in the first test tube in the image below.

Now Sodium iodide dissolves
in water to form a colourless solution containing sodium ions (Na+) and chloride ions (CI - ).
When chlorine water is added to the test-tube containing the sodium iodide solution and cyclohexane and then shaken for around 30 seconds you can see in the image above that the
cyclohexane layer has changed colour, it has turned a violet/purple colour and the sodium chloride solution has a brown colouring.
It may be easier to understand what is happening in this reaction if we write an equation for this displacement reaction that has taken place:
On the reactants side of the word equation we have iodide ions (I-) in the sodium iodide solution and chlorine from the chlorine water. In a displacement reaction the more reactive halogen will kick-out or displace the less reactive halogen from its compounds, in this case chlorine will displace iodide from the sodium iodide solution. The chlorine effectively takes the place of the iodide ions. The iodide ions are now kicked out of the solution and now have a choice of places to go to; either into the cyclohexane layer or the aqueous (water layer).
Since Iodine is more soluble in the
cyclohexane layer than in the aqueous (water) layer most of the iodine will dissolve in the cyclohexane turning it a vivid purple colour. Some "iodine" will also be dissolved in the aqueous layer turning it a light brown colour (in reality it is likely to be the presence of triodide ions in the solution that are actually responsible for the brown colour though for simplicity we can say that the brown colour is due to iodine!.
In the example shown in the image below we again have 1 halogen (chlorine) and a halide ion (bromide Br-) on the reactants side of the equation; these are the bromide ion ; in the form of from the sodium bromide solution and chlorine from chlorine water. Since chlorine is more reactive than bromine it will displace the bromide ion from the sodium bromide solution, the bromide will be kicked out of solution and most of it will then dissolve in the cyclohexane layer, turning it red/brown. However some bromine will also dissolve in the aqueous sodium chloride layer turning it a pale yellow colour.

Equations for this displacement reaction are:

Care is needed sometimes when considering halogen displacement reactions as you can get caught out quite easily! Consider the reaction shown on the right. Here we have a solution of sodium chloride in the boiling tube with iodine solution being added to it. Equations for this reaction are:
Answer the four questions in the drop-down boxes below to check your understanding of the main points on halogen displacement reactions outlined above. Simply click the blue coloured boxes to reveal the answers.
 The first four halogens are fluorine, chlorine, bromine and iodine. They are all found in group 7 of the periodic table and the order of reactivity is F, Cl , Br , I. Fluorine is the most reactive non-metal element in the periodic table and it is highly unlikely you will ever meet this element except perhaps under the most controlled conditions.
The first four halogens are fluorine, chlorine, bromine and iodine. They are all found in group 7 of the periodic table and the order of reactivity is F, Cl , Br , I. Fluorine is the most reactive non-metal element in the periodic table and it is highly unlikely you will ever meet this element except perhaps under the most controlled conditions.
 In a halogen displacement reaction a more reactive halogen will displace or kick-out a less reactive halogen atom or halide ion from its solution or compound. These reactions are a good way to demonstrate the order of reactivity of the halogens, for example:
In a halogen displacement reaction a more reactive halogen will displace or kick-out a less reactive halogen atom or halide ion from its solution or compound. These reactions are a good way to demonstrate the order of reactivity of the halogens, for example:
 The halogens are all soluble in organic solvents such as hexane or cyclohexane and they also give vivid clear colours when they are dissolved in these solvents. The halogens are not particularly soluble in water and give rather bland and washed out colours in aqueous solutions; for example:
The halogens are all soluble in organic solvents such as hexane or cyclohexane and they also give vivid clear colours when they are dissolved in these solvents. The halogens are not particularly soluble in water and give rather bland and washed out colours in aqueous solutions; for example:
 The halogens dissolve in water to form solutions with rather washed out colours especially when the solutions are dilute. Bromine for example dissolves to form a pale yellow solution when it is dilute but its colour changes tso red-orange as the concentration of bromine present increase. Iodine solution is pale brown when dilute but darkens considerable to form a dark brown solution as the concentration of the solution is increased. Chlorine solution is almost colourless when dilute but appears pale green when its concentration rises.
The halogens dissolve in water to form solutions with rather washed out colours especially when the solutions are dilute. Bromine for example dissolves to form a pale yellow solution when it is dilute but its colour changes tso red-orange as the concentration of bromine present increase. Iodine solution is pale brown when dilute but darkens considerable to form a dark brown solution as the concentration of the solution is increased. Chlorine solution is almost colourless when dilute but appears pale green when its concentration rises.You may recall that oxidation can be defined as a loss of electrons and reduction as a gain of electrons. Consider again the halogen displacement reaction which occurs when chlorine water is added to a sodium bromide solution. Now sodium bromide is an ionic compound containing sodium ions (Na+) and bromide ions (Br-).
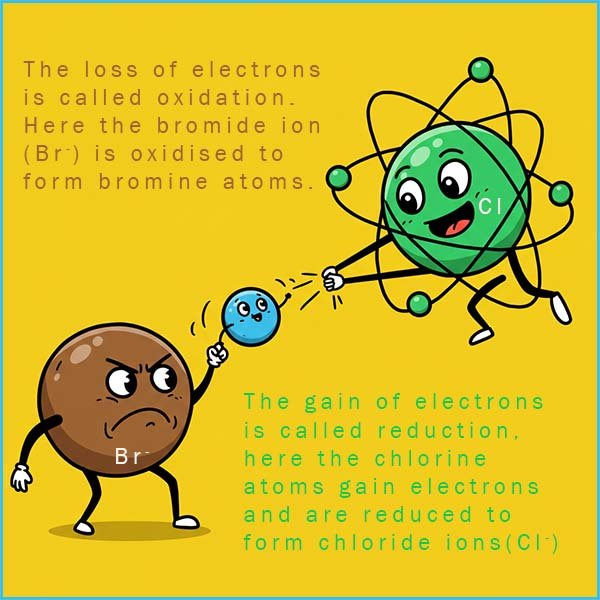
The product of this displacement reaction is sodium chloride and bromine. This means that as the reaction takes place the bromide ions (Br -) are being oxidised, that is they are losing electrons to form bromine atoms and the chlorine atoms are gaining electrons or being reduced to form chloride ions (Cl-). We can show this as:
Both of these equations needs to be multiplied by two since the halogens exits as diatomic molecules. So as well as being a halogen displacement reaction the reaction of sodium bromide and chlorine is also a redox reaction, where the more reactive halogen oxidises the less reactive halide ion. The more reactive halogen is acting an oxidising agent (electron acceptor) the less reactive halogen is acting as a reducing agent (electron donor).
Use the flashcards below to review and test your understanding of halogen displacement reactions: