

Higher and foundation tiers
The periodic table contains all the known elements. The periodic table shown below
contains all the elements up
to element 88, radium. The elements are arranged in the periodic table in vertical columns called groups and horizontal rows called periods as shown in the image below. You should be aware that:
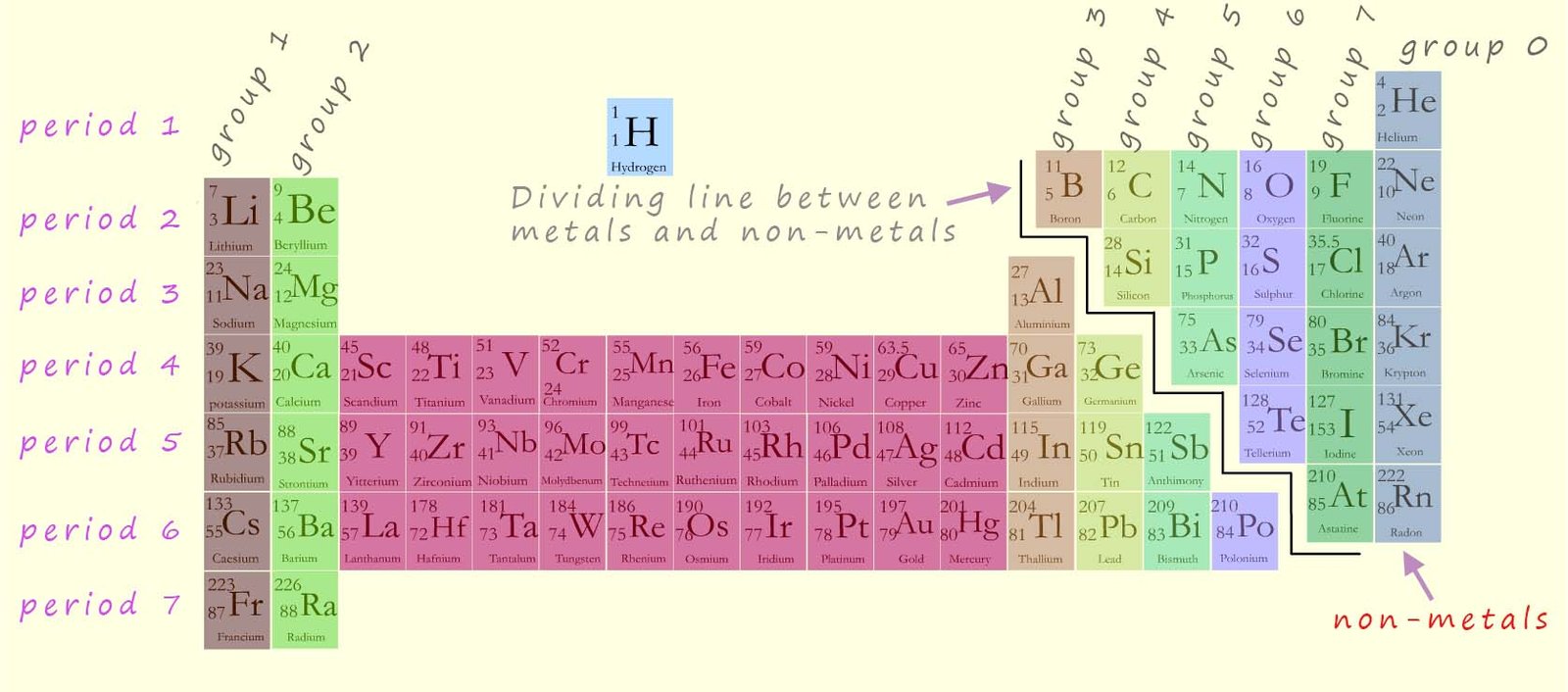
Since the in which way any particular element reacts depends entirely on the number of electrons in the outer electron shell or valency shell; this means that all the elements in any one particular group in the periodic table will react in a similar way, since each element in any one group will have the same number of electrons in their outer electron shell or energy level. The elements in any one group will obviously not react in an identical way since they have different numbers of electrons.
The central block of the periodic table contains the transition metals (shown in red in the image above), however knowledge of the chemistry of these metals is not required at GCSE level but is covered in the A-level chemistry course.
The modern version of the periodic table is of great value to scientists.
In the modern periodic table the elements are arranged according to
their atomic number and arranged in vertical columns or groups according to the number of electrons present in their outer electron shells and on their chemical
properties and reactions. This enables
scientists to predict and work out how any one element will react and behave even if they have never
seen or used this element before. However it took many years to arrive at this version of the
periodic
table and early versions of the periodic table were very different
to the one you are familiar with today.
You may have wondered why the periodic table is the shape it is, why not arrange the elements in say a square, a rectangle or even a circle! Well believe it or not once you get to know your way around the periodic table you will quickly realise why it is such a great asset to any chemistry student and why it is set out the way it is.
Imagine for a moment you were starting to build a jigsaw but without a picture of what you are trying to build. You might start with all the edge pieces or put all the pieces of say the sky together or any pieces of say a building that you thought might be in the jigsaw picture.

Well when trying to construct the periodic table over 150 years ago scientists faced a similar problem. New elements were being discovered all the time, they had some limited information about these newly discovered elements; some of which was correct and some incorrect and of course they knew nothing about protons, neutrons or electrons or our ideas about the structure of atoms.

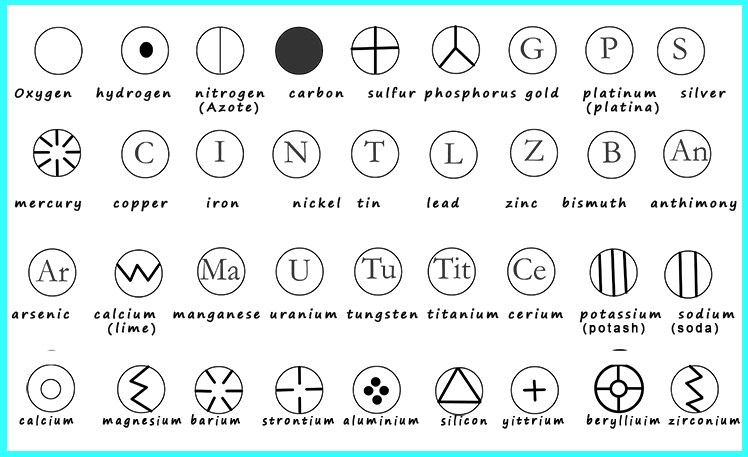
In 1803, John Dalton an English School teacher who was interested in physics, meteorology and chemistry (atomic theory) produced a periodic table of sorts. Dalton's atomic theory which he used to construct his periodic table suggested that:
Over 20 years later Dalton published a revised volume of work which contained his periodic table of elements; in fact some of his elements were actually compounds! A revised version of John Dalton's periodic table is shown below. His symbols may look a little odd when compared to those used today and they are a little difficult to remember which is why they have not been used since. But it was a start at constructing a periodic table and trying to group the elements together in some way and spot patterns in their chemical reactions.
 A German chemist named Johann Wolfgang Döbereiner made some interesting observations in 1829
which would help chemists in developing the periodic table; but his ideas were not given enough weight
and dismissed as a curiosity or coincidence. Döbereiner noticed that the properties of the element bromine
seemed similar to those of chlorine and iodine. Now
bromine is a liquid at room temperature and he placed it in one of his triads between chlorine;
a gas and iodine; a solid. Döbereiner also noticed that the mass of bromine was
approximately half-way
between that of the element above it and the one below
it- chlorine and iodine
A German chemist named Johann Wolfgang Döbereiner made some interesting observations in 1829
which would help chemists in developing the periodic table; but his ideas were not given enough weight
and dismissed as a curiosity or coincidence. Döbereiner noticed that the properties of the element bromine
seemed similar to those of chlorine and iodine. Now
bromine is a liquid at room temperature and he placed it in one of his triads between chlorine;
a gas and iodine; a solid. Döbereiner also noticed that the mass of bromine was
approximately half-way
between that of the element above it and the one below
it- chlorine and iodine
Döbereiner also noticed similar patterns in the masses of the three elements calcium (Ca), Strontium (Sr) and barium (Ba), as well as sulfur (S), selenium (Se) and tellurium (Te). These triads (groups of three elements) with the patterns in their atomic masses and similar chemical reactions of the three elements in the triad should have helped scientists to construct a periodic table.
In 1864 an English chemist named John Newlands published his periodic table, while it had many limitations it was one of the first attempts at arranging the known elements both in terms of their atomic weights (which we now call relative atomic mass) and their chemical properties, but perhaps the most significant discovery for Newlands was his "Law of Octaves".
Newlands took all the known elements (around 50 in 1865) and arranged them in order of their atomic mass; as shown in his periodic table below. He suggested that every eighth element in his periodic table had similar chemical properties e.g. starting with hydrogen and counting 8 we come to the element fluorine. The eighth element after fluorine is chlorine and so on.... However his observation that every eighth element had similar chemical properties only worked for the first 20 elements; after that it failed. For example the elements lithium, sodium and potassium all react violently with water but Newlands put the element copper, which does NOT react with water in the same group as these reactive elements.
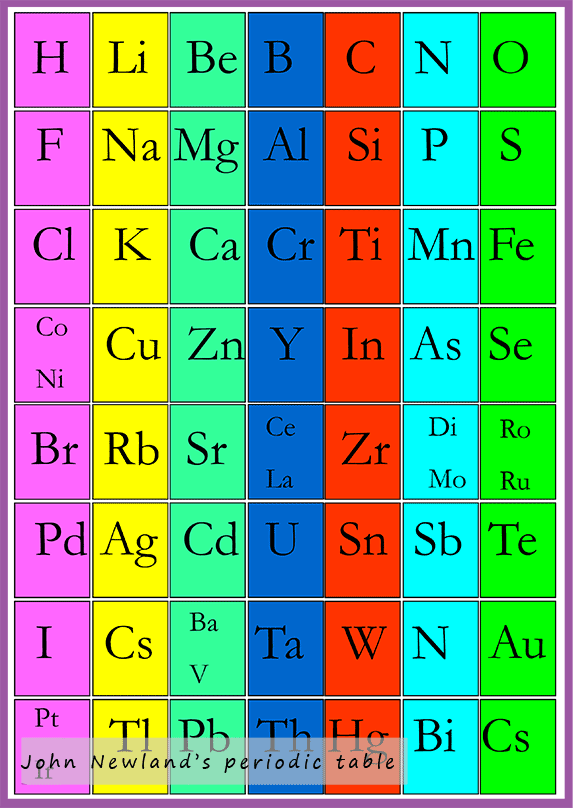
There were other problems with Newland's his periodic table. In places Newlands had more than one element in a box in his periodic table and he put elements such as iron in the same group as oxygen and sulfur when these elements have little in common. Newlands grouped these elements together in order to get them to fit his "Law of octaves" even though it was apparent that some elements in his groups were not similar at all, unfortunately for Newland's this was one of the main reasons why his periodic table was not immediately accepted by his peers. One of the other big mistakes he made in constructing his periodic table was that he assumed that all the elements had been discovered when in reality new elements were being discovered on a fairly regularly. His ideas were rejected by his peers and he was even ridiculed by some but his periodic table paved the way for Dmitri Mendeleev and his periodic table. The noble gases had not been discovered and so were unknown to Newlands when he devised his periodic table, however with their discovery an extra column would be needed in the periodic table and this would have completely disrupted his Law of octaves.
In 1869 the Russian scientist Dmitri Mendeleev improved on Newlands periodic table. Like Newlands he arranged the elements in order of their atomic masses and he grouped elements with similar chemical properties together in their own groups, but unlike Newlands he assumed that there were still elements to be discovered and he left gaps for these undiscovered elements in his periodic table.
Not only that, he predicted the properties of some of these as yet undiscovered elements based on their position in his periodic table. When these unknown elements were finally discovered it was found that their properties were very similar to those predicted by Mendeleev.
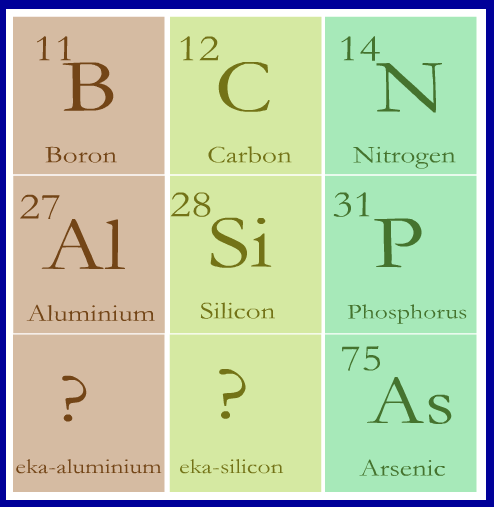
Part of Mendeleev's periodic table is shown opposite. It contains
the elements boron (B) and aluminium (Al) in one group together with their atomic masses. Underneath aluminium Mendeleev left a gap
in his periodic table for an element that he believed was as yet undiscovered. He named this undiscovered element eka-aluminium.
Similarly underneath carbon (C) and silicon (Si) was yet another undiscovered element that Mendeleev named
eka-silicon.
In 1875 the element gallium (Mendeleev's eka-aluminium) was discovered and in 1886 the element
germanium (Mendeleev's eka-silicon) was discovered. These two new elements had
chemical and physical
properties that were very close to Mendeleev's predicted properties. This suggested that Mendeleev's hypothesis and
ideas on the structure of the periodic table were probably correct.
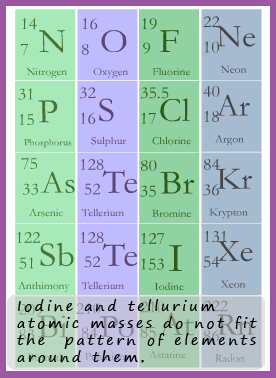
It was not until much later following the work of scientists such Rutherford and Henry Moseley that the idea of atomic number instead of atomic mass as a way of arranging the elements that anomalies like the tellurium/iodine one were eventually solved. The modern periodic table we use today sorts the elements according to their atomic number and not their atomic mass.
Match the terms below with the correct definitions by simply clicking on the term and then its correct definition. Correct responses will turn the definition green!
Use the flashcards below to check your understanding of the main ideas covered in this page.