

Foundation and higher tiers
The positively charged metal ions and negatively charged non-metal ions produced when
metals and non-metals react with each other to form ionic compounds, these oppositely charged ions will be attracted to each
other and pack as closely together as possible in a regular ordered way. Structures with regular arrangements of atoms or ions
are often referred to as being crystalline.
Sodium a group I alkali metal will form an ion with a +1 charge when it reacts with a non-metal such as chlorine, while chlorine a group 7 halogen will form an ion with a -1 charge, when it reacts with a metal such as sodium. If you were to look at grains of sodium chloride or salt; an ionic compound formed when sodium and chlorine react; under the microscope you will see that they have a cubic shape. The image below shows the structure of the sodium chloride lattice. The green spheres represent chloride ions (Cl-) and the blue spheres sodium ions (Na+).

 If you were to count the ions in the model opposite you would notice that
there are equal numbers of positively charged sodium ions (Na+) and
negatively charged chloride ions (Cl-). This is simply because when the ions pack together they do in
such a way that the charges always
cancel out. The +1 charge on the sodium ion is cancelled out by
the -1 charge on the
chloride ion. So the ratio of
each ion present in the sodium chloride lattice is 1:1.
If you were to count the ions in the model opposite you would notice that
there are equal numbers of positively charged sodium ions (Na+) and
negatively charged chloride ions (Cl-). This is simply because when the ions pack together they do in
such a way that the charges always
cancel out. The +1 charge on the sodium ion is cancelled out by
the -1 charge on the
chloride ion. So the ratio of
each ion present in the sodium chloride lattice is 1:1.
This simple ratio of ions enables you to calculate the empirical (simplest) formula for the compound.
So in the case of sodium chloride it is NaCl. Its ionic formula; which is simply the chemical formula with the charges on the ions shown is simply Na+Cl-.
You will often see the structure of the giant lattice structure for ionic compounds shown in different ways. This is to try and help you
visualise what the giant structure looks like. The image opposite shows the cubic lattice for sodium chloride (NaCl). The chloride ions (Cl-) are shown in
green and the sodium ions (Na+) are a yellow/green colour; but this time there are no bonds shown between the ions. In reality
there would be no gaps between the ions as they will pack together as closely as possible but this model does allow you to view
the interior of the structure which is not possible from the first model. Both models should help you to visualise how the ions
are arranged in the giant lattice structure.
Use the flashcards below to review your understanding of the key points on ionic compounds mentioned above. Click the flashcards to reveal the answers.




 None of the models for sodium chloride shown allow
you to spot immediately how the crystal structure is built. If
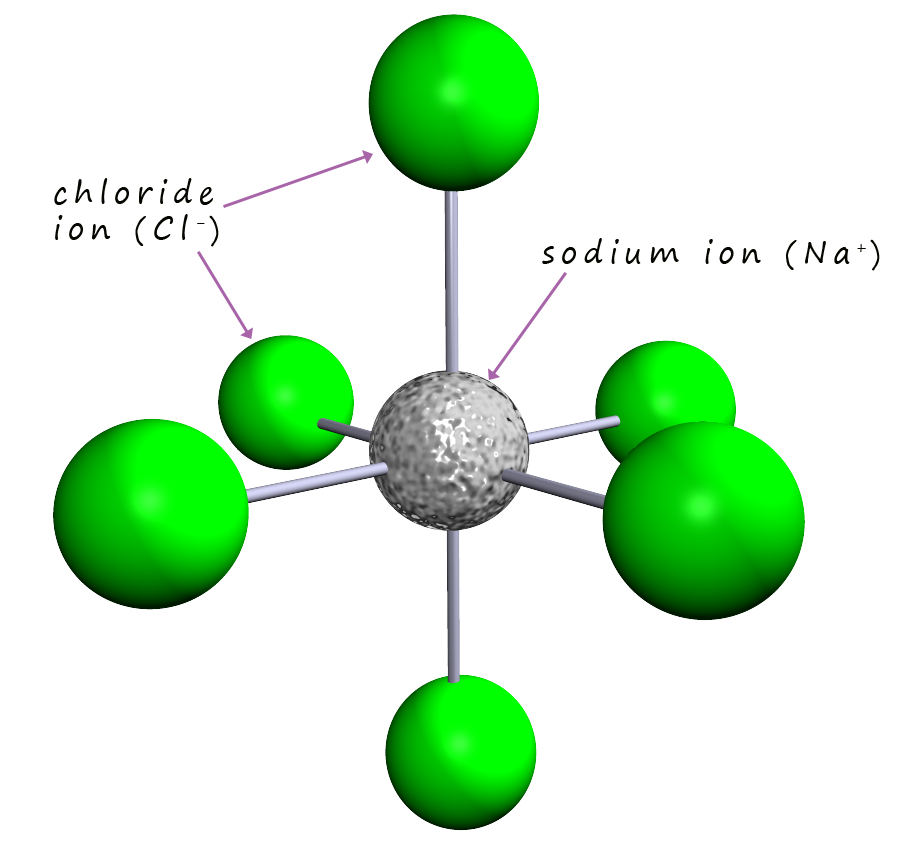
you pick out one sodium ion you should see that it has six immediate neighbours. One
chloride above it and one below;
one to its left and one to its right and one in front and one behind. This is shown in the image opposite. The same is true if you
pick a chloride ion. Each sodium or chloride
ion has 6 immediate neighbours; the ions within this lattice structure are described as six coordinate.
None of the models for sodium chloride shown allow
you to spot immediately how the crystal structure is built. If
you pick out one sodium ion you should see that it has six immediate neighbours. One
chloride above it and one below;
one to its left and one to its right and one in front and one behind. This is shown in the image opposite. The same is true if you
pick a chloride ion. Each sodium or chloride
ion has 6 immediate neighbours; the ions within this lattice structure are described as six coordinate.
All ionic compounds have a giant lattice structure made up of ions. However not all ionic lattices have
a face centred cubic structure like sodium chloride or are six co-ordinate. Recall that the ions in an ionic lattice will always try and pack together
as closely as possible but in a way that maximises the attraction between oppositely
charged ions and
reduces the repulsion between ions of a similar charge.
However the differences in the sizes of the ions present in the
ionic compound will limit how closely they can pack together to maximise attractions and reduce repulsion. The image below shows
ball and stick models for the structure of the calcium fluoride lattice. Clearly it is not cubic like the sodium
chloride lattice, this is simply because the ions present are different in size and charge from those present in sodium chloride. In the models the red ions are Ca2+ and the blue
ions are F-.

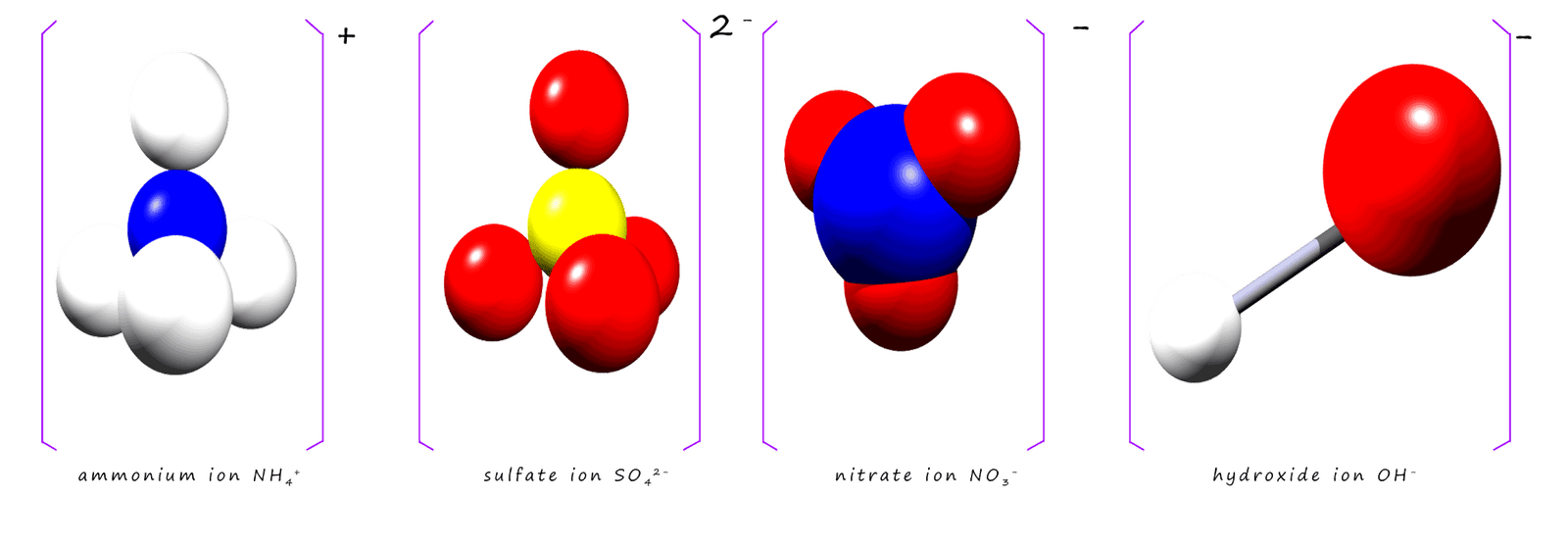
Some ions are compounds and contain more than one type of element. You will likely have met these ions in the past but perhaps not realised they were group ions. For example the sulfate ion (SO42-), ammonium ion (NH4+) and hydroxide ion (OH-) are all compounds and also ions which contain more than one element. These ions are often called polyatomic ions or simply group ions, a few common polyatomic or group ions are shown in the image below:
 When working out the formula of compounds containing these ions just use the same rules as before e.g.
When working out the formula of compounds containing these ions just use the same rules as before e.g.
Use the flashcards below to work out the formula for a few ionic compounds. Click the flashcards to reveal the answers.





