

Foundation and higher tiers

Ionic compounds all have a giant ionic lattice structure. The ions within the giant ionic lattice are held in fixed positions and so cannot move freely since they are held in place by strong Ionic bonds. However most ionic compounds will dissolve in water; especially those containing a metal ion from group I and the bottom end of group II in the periodic table.
When an ionic compound dissolves in water the water molecules will pull apart the giant lattice structure and we end up with ions which are separate from each other and which are able to move freely within the solution formed. If an electrical current is passed through this solution it will readily flow- solutions of ionic compounds conduct electricity, these solutions are often called electrolytes. This is why for example you can get a nasty electrical shock if you accidentally touch a live electrical wire with wet hands. Since you sweat salt water; which is a solution of sodium chloride; then this solution will conduct electricity and result in a severe electrical shock.
The image below shows the cubic structure of the sodium chloride ionic lattice. When sodium chloride or table salt is added to water and stirred it will dissolve. The water molecules are able to dismantle the giant ionic lattice by pulling out the sodium and chloride ions, these ions are now able to move freely within the solution; (this is outlined in more detail below). These solutions of ions as mentioned above are often referred to as electrolytes and they are good conductors of electricity.


There are a few simple guidelines that can be used to help you decide in advance if an ionic compound will be soluble in water or not; the following points should help you in deciding if an ionic compound is soluble or not:
The image below shows the apparatus which can be used to demonstrate the fact that solutions of ionic compounds will conduct electricity. Here two graphite electrodes are dipped into a solution of an ionic compound; the free moving positive and negatively charged ions present in the solution will be attracted towards the electrodes. The positively charged metal ions will be attracted to the negatively charged electrode (the cathode) and the negatively charged non-metal ions in the solution will be attracted to the positively charged electrode (the anode). These free moving ions are able to conduct an electrical current and this explains why the bulb in the image below lights up.

Many ionic compounds are soluble in water, a phrase which is often used when discussing solubility is "like dissolves like". What this means is that ionic lattices obviously consist of positively charged metal ions and negatively charged non-metal ions, well water can use its lone pairs of electrons to form intermolecular bonds with the positively charged metal ions in the lattice and effective pull the metal ions out of the ionic lattice and into solution. The water molecules can also form intermolecular bonds to the chloride ions (Cl-) and pull these from the ionic lattice and into solution as well. The ions are said to be solvated or hydrated, this process is shown in the image below:

If a solid ionic compound is heated to a enough high temperature it melts to form a liquid or a melt. This molten liquid or melt will also conduct electricity; since the giant ionic lattice structure will break down during the melting process. The ions present in this liquid or melt are free to move and these free moving ions just as in the aqueous solution above they are able to conduct an electrical current, since it is the presence of free moving ions that enable an electric current to flow in solutions and melts. It therefore makes sense that solid ionic compounds DO NOT conduct electricity since the ions are held tightly in a 3d lattice structure and are not free to move.

Ionic compounds have giant structures
with lots of strong ionic bonds between the ions, it takes a lot of energy to
break all these strong bonds and so ionic compounds have very
high melting and boiling points.
Sodium chloride (Na+Cl-) for example has a melting point of 8010C. Aluminium oxide (Al2O3) has
metal ions with a much larger charge than the sodium ions (Na+) in sodium chloride. Aluminium is a group 3 metal so its ion will have a 3+ charge (Al3+) and the oxide ions (O2-) will have a charge of negative two (O2-). The oxide ions are also much smaller than the chloride ions in
sodium chloride. The larger charges and smaller sizes of the aluminium and oxide ions means that the
ions can pack together very closely and the
attraction between the ions is also much larger; so aluminium oxide has a very high melting point (20720C).
Use the flashcards below to quickly test your understanding of the main points covered above. Simply click the flashcards to reveal the answers.





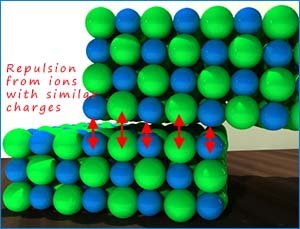
If an ionic lattice is subjected to any pushing or pulling forces
which causes the layers of ions to move this will lead to
widespread cracking within the lattice structure; as ions
of similar charge are brought in contact with each other. This will cause the
ions to immediately repel each other and the
lattice structure will break apart at this point. This means that ionic lattices are
brittle
and can easily break. This is shown in the image below where ions of similar charge sit on top of one another; this will cause
repulsion and the ionic lattice will fracture at these points.

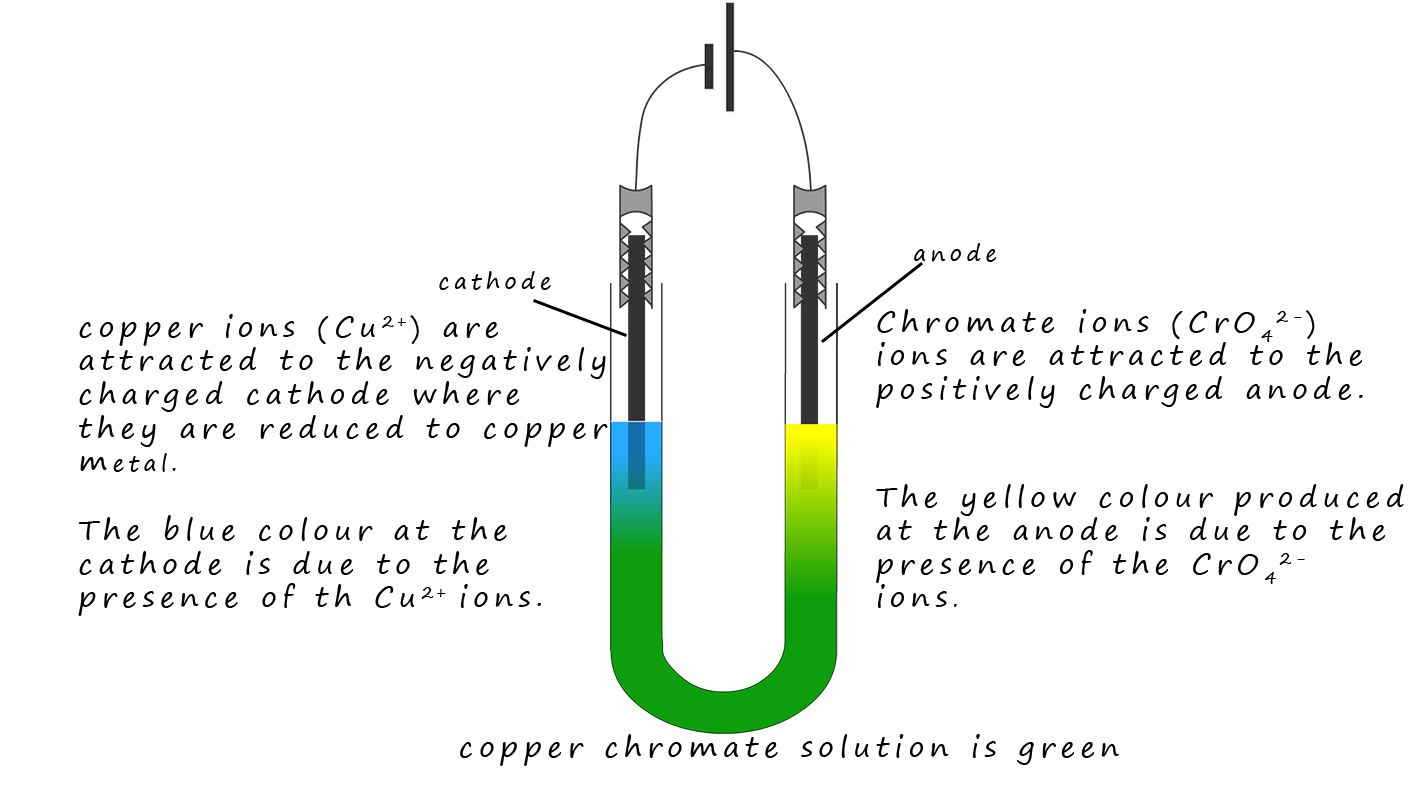 Copper chromate is an ionic compound containing blue copper ions (Cu2+) ions and yellow chromate ions
(CrO42-). These two coloured ions
mix and the resulting solution is green. However when the solution is
electrolysed the positively charged
blue (Cu2+) ions are attracted to the negatively charged cathode and the
yellow CrO42- ions are attracted to the positively charged anode. This simple
demonstration is a good piece of evidence for the presence of ions in a solution.
Copper chromate is an ionic compound containing blue copper ions (Cu2+) ions and yellow chromate ions
(CrO42-). These two coloured ions
mix and the resulting solution is green. However when the solution is
electrolysed the positively charged
blue (Cu2+) ions are attracted to the negatively charged cathode and the
yellow CrO42- ions are attracted to the positively charged anode. This simple
demonstration is a good piece of evidence for the presence of ions in a solution.
Answer the two questions below to review some of the key points from above:
 When ionic lattices are subjected to pushing or pulling forces some of the layers of ions in the ionic lattice can be shifted and move, this may cause ions of similar charge to come into contact with each other, they will then immediately repel each other, this will cause the lattice to crack and break apart.
When ionic lattices are subjected to pushing or pulling forces some of the layers of ions in the ionic lattice can be shifted and move, this may cause ions of similar charge to come into contact with each other, they will then immediately repel each other, this will cause the lattice to crack and break apart.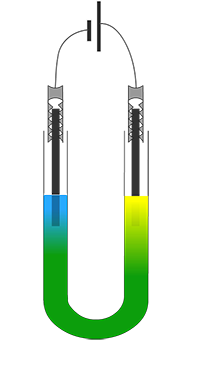 The green colour of a copper chromate solution is due to the presence of blue copper ions (Cu2+) and yellow chromate ions (CrO42-) ions. The blue and yellow colours combine to produce a yellow coloured solution.
When this solution is electrolysed the blue copper ions (Cu2+) will be attracted to the cathode where they will be reduced to form brown copper metal; which will plate the cathode.
The green colour of a copper chromate solution is due to the presence of blue copper ions (Cu2+) and yellow chromate ions (CrO42-) ions. The blue and yellow colours combine to produce a yellow coloured solution.
When this solution is electrolysed the blue copper ions (Cu2+) will be attracted to the cathode where they will be reduced to form brown copper metal; which will plate the cathode.
