

This page introduces the functional groups you are most likely to meet in your A-level chemistry course and gives you clear advice and tips on how to name molecules containing these different functional groups. Feel free to scroll through the page, or use the menu above to jump straight to a particular homologous series of compounds.
One of the thrills in organic chemistry is the skill and enjoyment from figuring out how to build a new
molecule from
simple starting reagents. To be able to build large molecules
from smaller ones you need to be able to predict how different
reactants will interact with each other and to do this you
need to be able to identify functional groups within
molecules and be able to predict how they will react with other substances.
Aliphatic compounds are organic compounds that contain carbon and hydrogen atoms connected in straight chains, branched chains and rings (but NOT aromatic rings). In year 12 almost all the molecules you will study in organic chemistry will be aliphatic, you may meet some cyclic molecules which consist of closed rings of atoms, but these rings are unlikely to be based on benzene. Compounds which contain benzene rings are often described as being aromatic and the chemistry of these aromatic molecules is considered mainly in A2 chemistry. The rest of this page reviews the functional groups you are likely to meet in your A-level chemistry course.
There are probably hundreds of thousands or even millions of organic compounds and to help identify and name them they are placed in families which form a homologous series of compounds. A homologous series is a series of compounds which contain the same functional group and the molecular formula of all the compounds in any one particular homologous series can be represented by a general formula.
As an example of a homologous series you have met before consider the alkanes. The alkanes form a homologous series of saturated hydrocarbons with the general formula (CnH2n+2). All alkanes contain single covalent bonds between all the bonded atoms in the molecule, that is all the C-C and C-H bonds. The first four alkanes are shown in the table below:
| Alkane | Number of carbon atoms | Molecular Formula | Structure |
|---|---|---|---|
| methane | 1 | CH4 | 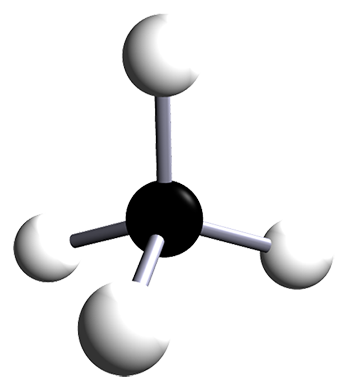 |
| ethane | 2 | C2H6 | 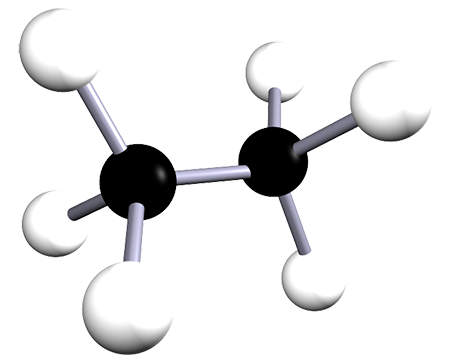 |
| propane | 3 | C3H8 |  |
| butane | 4 | C4H10 | 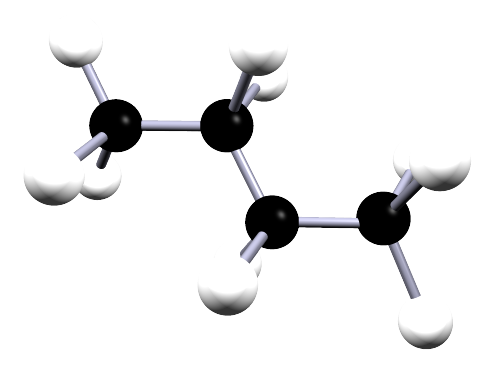 |
The alkane family is probably one of the first homologous series you learned about in chemistry, however there are many more; some of these are shown below:
| Homologous series | Name | Functional group | general formula |
|---|---|---|---|
| alkenes | suffix -ene | 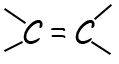 |
CnH2n |
The first 3 members of the alkene homologous series are shown below:
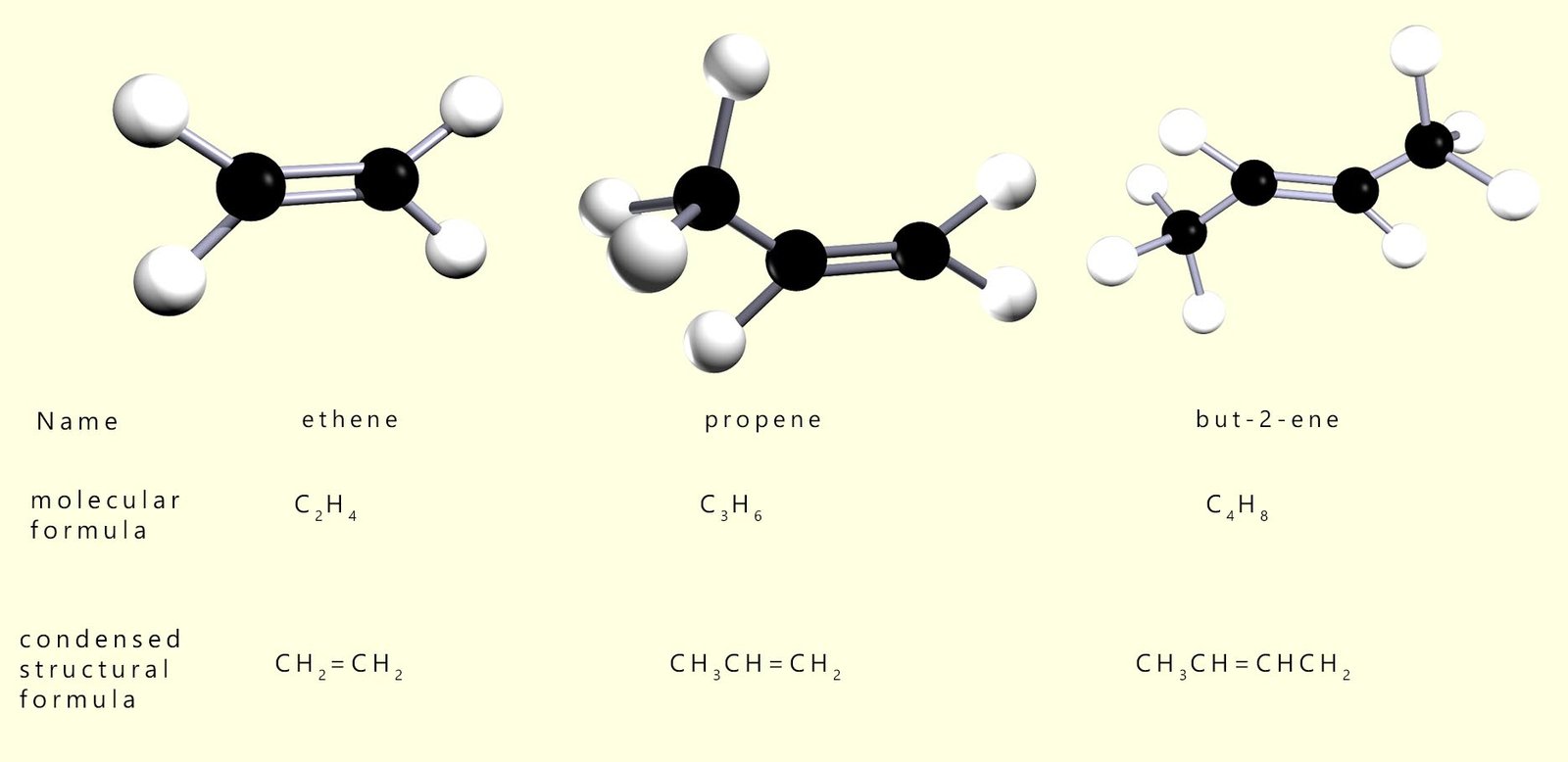
The alkenes are
all unsaturated
hydrocarbons, which means that they contain at least one carbon–carbon double bond (C=C).
This C=C bond is the reactive group, or functional group, present in all
alkene molecules.
Alkenes are named by first identifying the
longest carbon chain that contains the C=C
functional group.
The name of this parent chain forms the root of the compound and the suffix -ene is added to show the presence of a carbon–carbon double bond.
In many cases the position of the C=C bond must also be specified.
This is done by numbering the carbon atoms in the
longest carbon chain so that the double bond is given the lowest possible number.
The number used in the name tells you where the C=C
functional group starts along the chain.
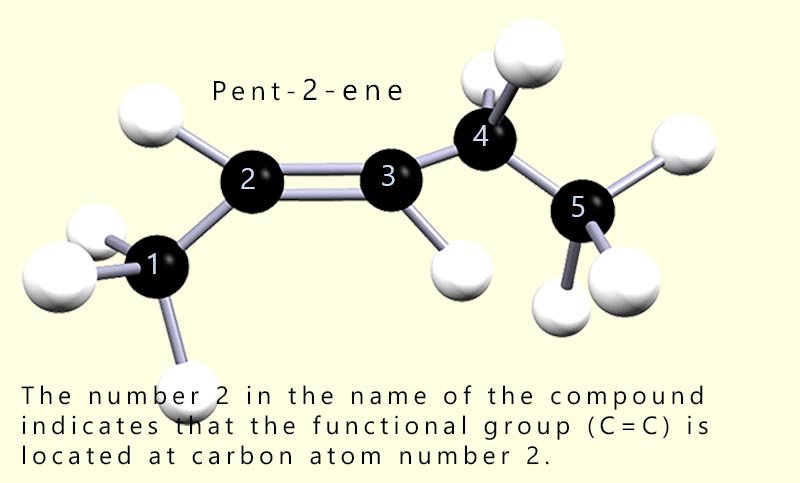
For example in the alkene pent-2-ene (shown opposite) the number 2 shows that the carbon–carbon double bond begins at the second carbon atom in the five-carbon chain.
This method ensures that different alkene molecules can be named clearly and unambiguously.
Study the alkene shown below and answer each step carefully, exactly as you would in an exam. The steps are designed to help you name this alkene.
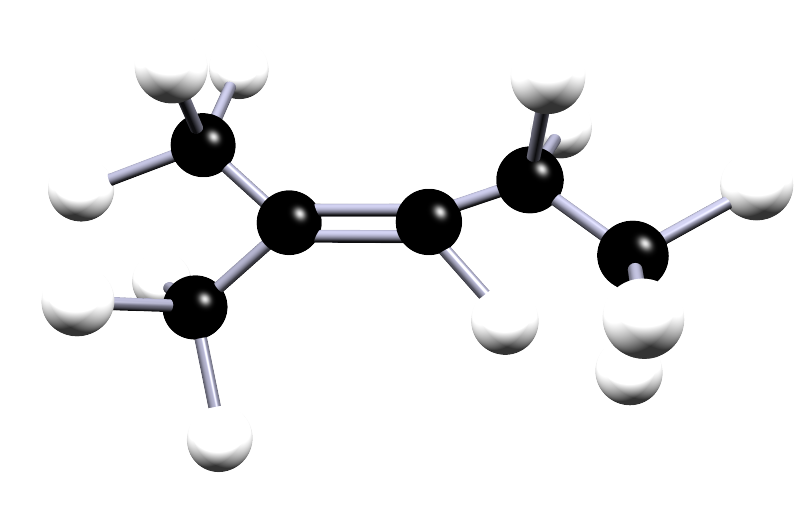
Step 1 – Identify the parent chain
What is the name of the longest carbon chain that contains the C=C bond?
Step 2 – Number the chain
From which end should the chain be numbered?
Step 3 – Locate the double bond
Step 4 – Identify substituents
Step 5 – Write the full name
Exam tip: always number the chain so the C=C bond gets the lowest possible number, even if a substituent ends up with the same number.
The halogenalkanes are another homologous series you may have met before. They are formed by replacing one or more of the hydrogen atoms in an alkane by a halogen.
| Homologous series | Name | Functional group | general formula |
|---|---|---|---|
| halogenalkane | prefix - halo. The halogen/halo can be fluoro, chloro, bromo, iodo |
C-F, C-Cl, C-Br, C-I or simply C-X |
CnH2n+1X |
| Order of priority |
|---|
| carboxylic acid |
| ester |
| nitrile |
| aldehyde |
| ketone |
| alcohol |
| amine |
| alkene |
| halogen |
Halogenalkanes are named in a similar way to the alkanes:
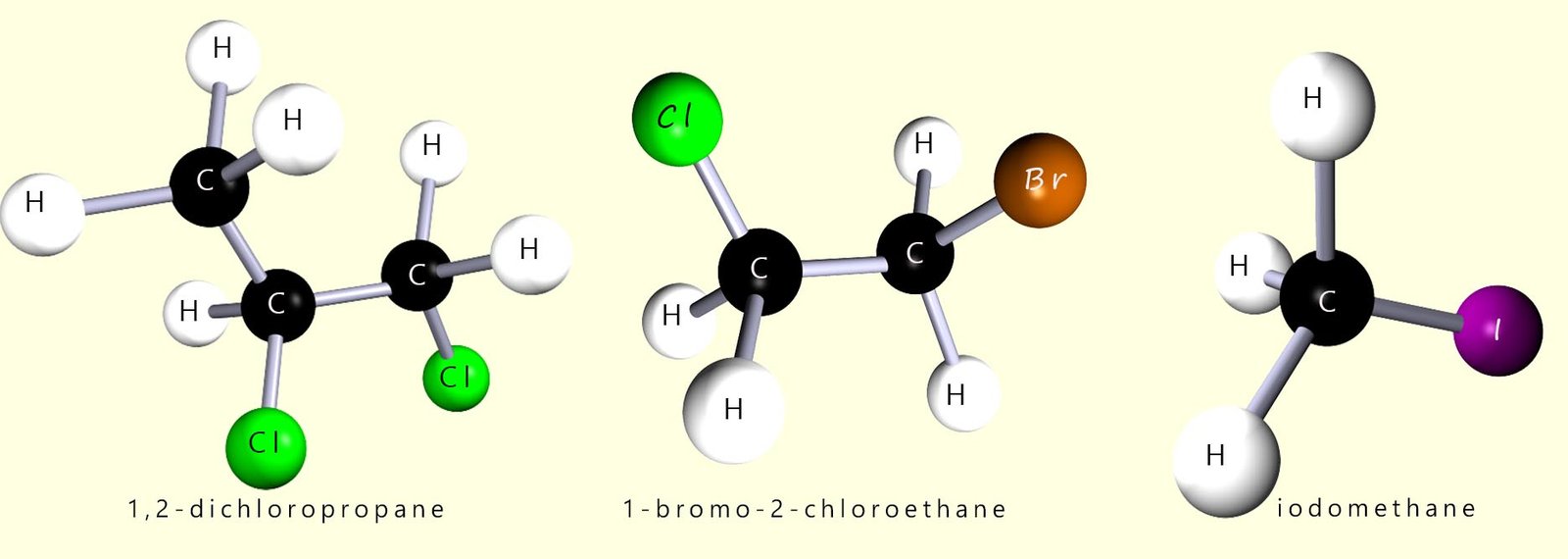
Some examples of simple halogenalkane molecules are shown below:
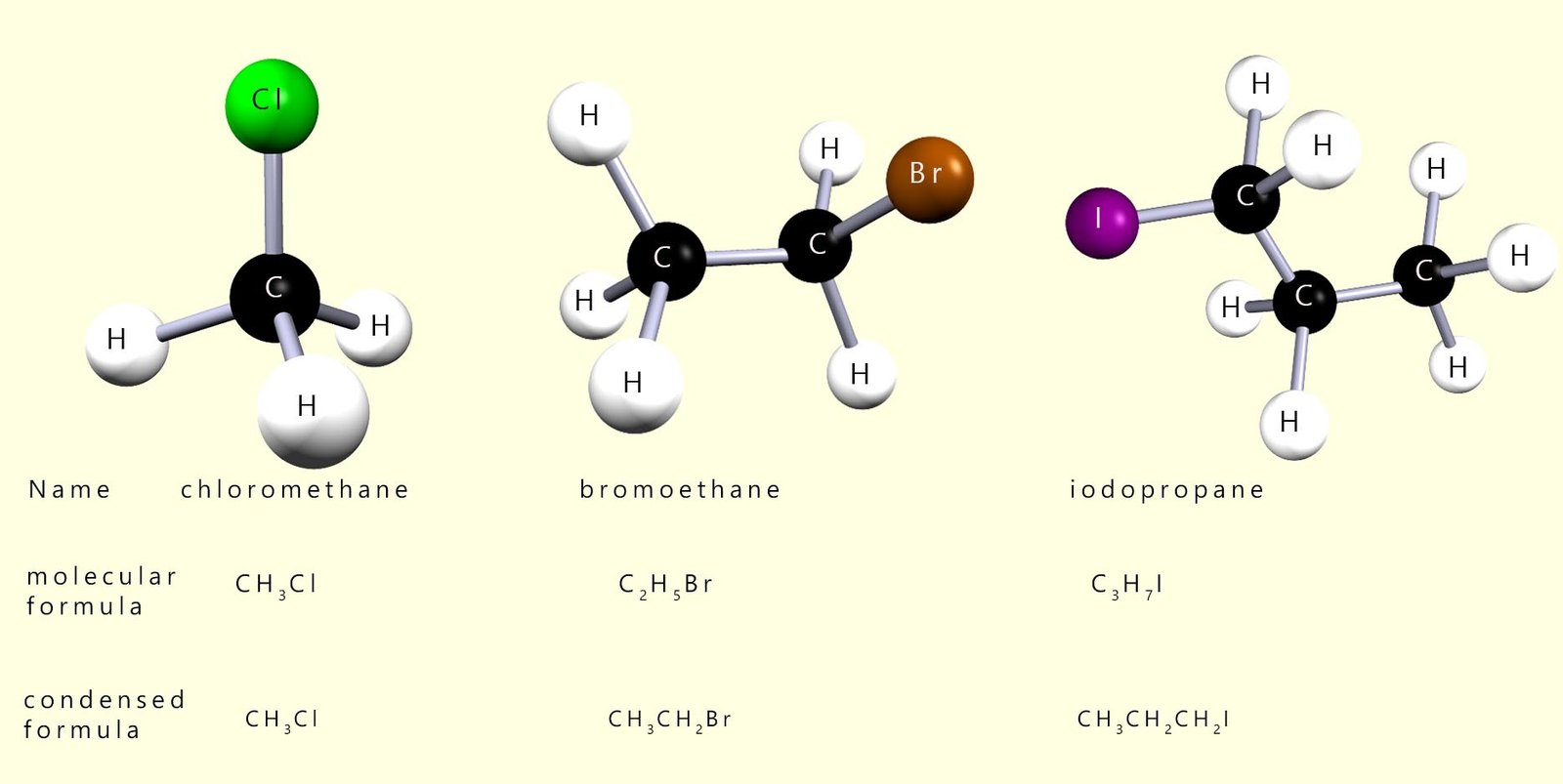
If more than one halogen is present then the positions and names are listed alphabetically, recall that when naming compounds if you insert numbers into the name then use commas between numbers and hyphens are used between numbers and letters e.g.
Use the information you have learned so far to name the halogenalkane below; especially those in the 5 bullet points above; simply click the button below to start.
Study the halogenalkane shown below and answer each step carefully, exactly as you would in an exam.
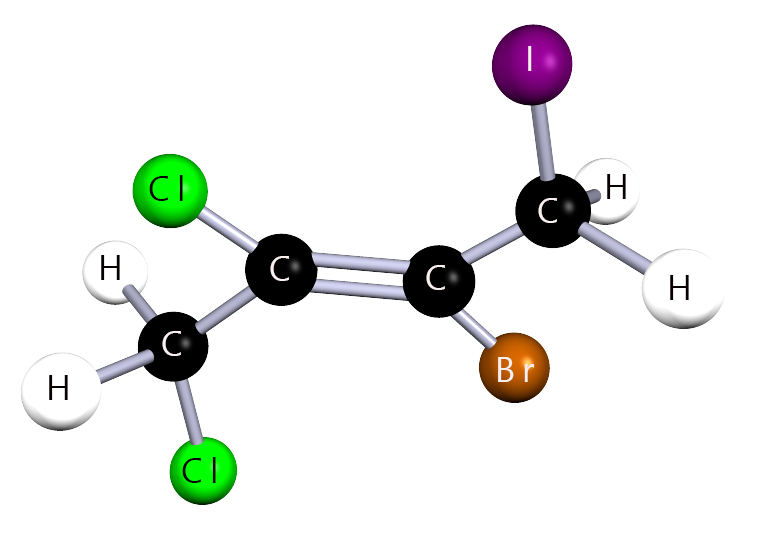
Step 1 – Identify the parent chain
What is the name of the longest continuous carbon chain?
Step 2 – Identify the substituents
Which halogen atoms are present in this molecule?
Step 3 – Number the carbon chain
From which end should the chain be numbered?
Step 4 – Write the name
Write the full IUPAC name, listing substituents in alphabetical order.
Exam tip: use commas between numbers, hyphens between numbers and words, and list substituents alphabetically (ignoring di-, tri-).
I suspect that some people found it difficult to name the molecule in the self-check activity above. The image below shows the molecule in question with the two most probable names that students would probably give it. The actual name of this molecule is 2-bromo-3,4-dichloro-1-iodobut-2-ene . To name it properly you must follow the rules in the 5 bullet points listed above.
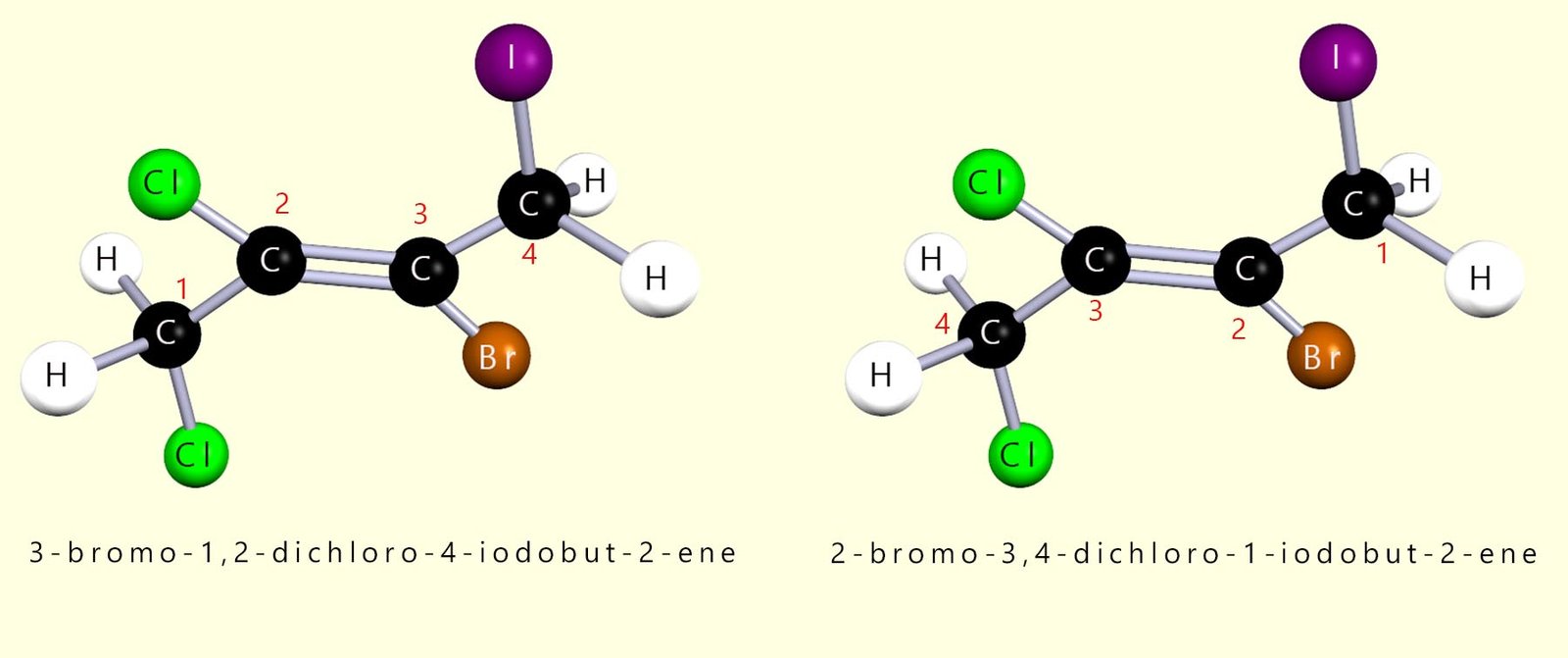
So if we apply the rules above then we have:
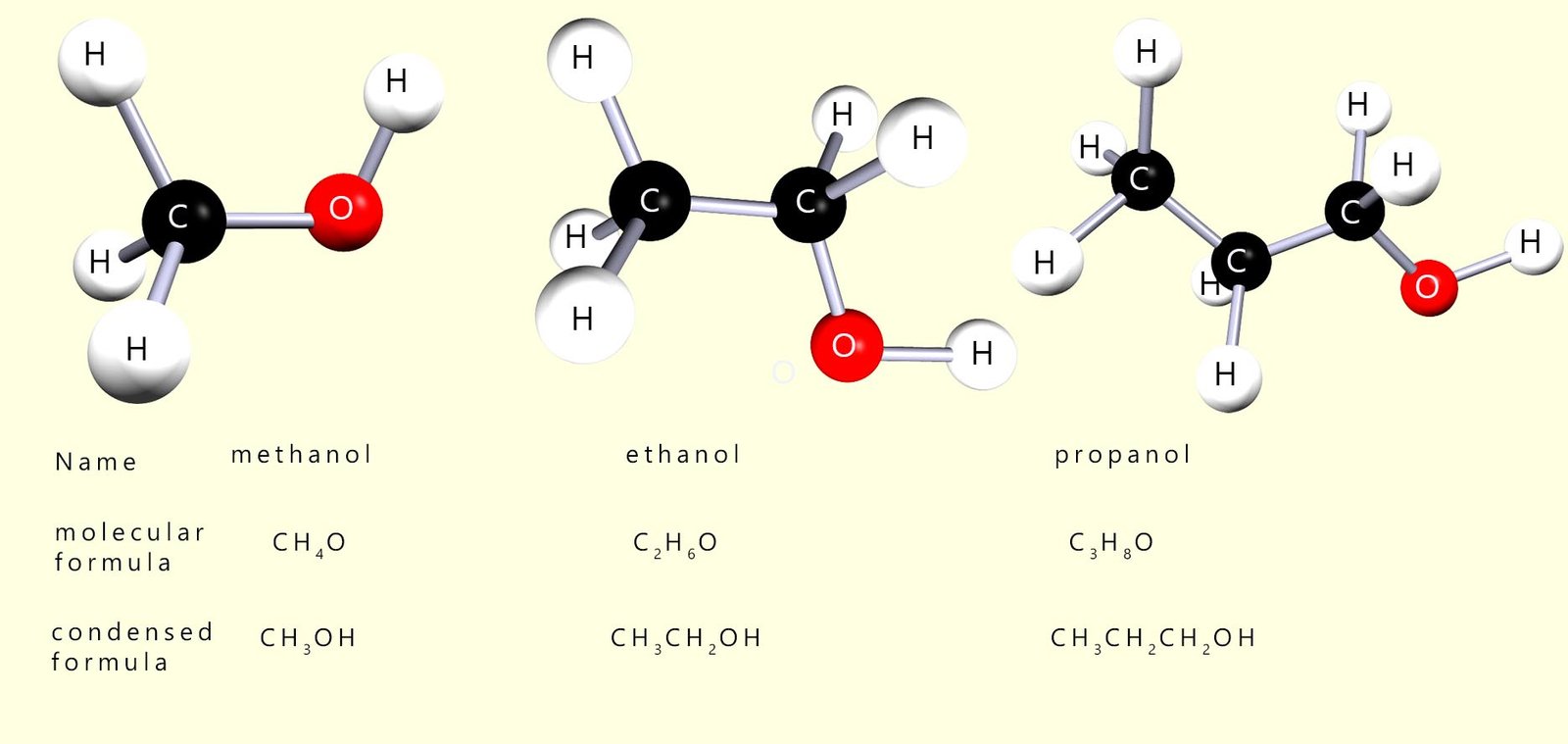
The alcohols are a family of organic compounds which all contain the hydroxyl functional group (R-OH). The first 3 members of this homologous series are shown in the image below:
| Homologous series | Name | Functional group | general formula |
|---|---|---|---|
| alcohols | suffix -ol prefix -hydroxy |
C-OH | CnH2n+1 OH |
Alcohols are named as being derived from alkanes. To name an alcohol:
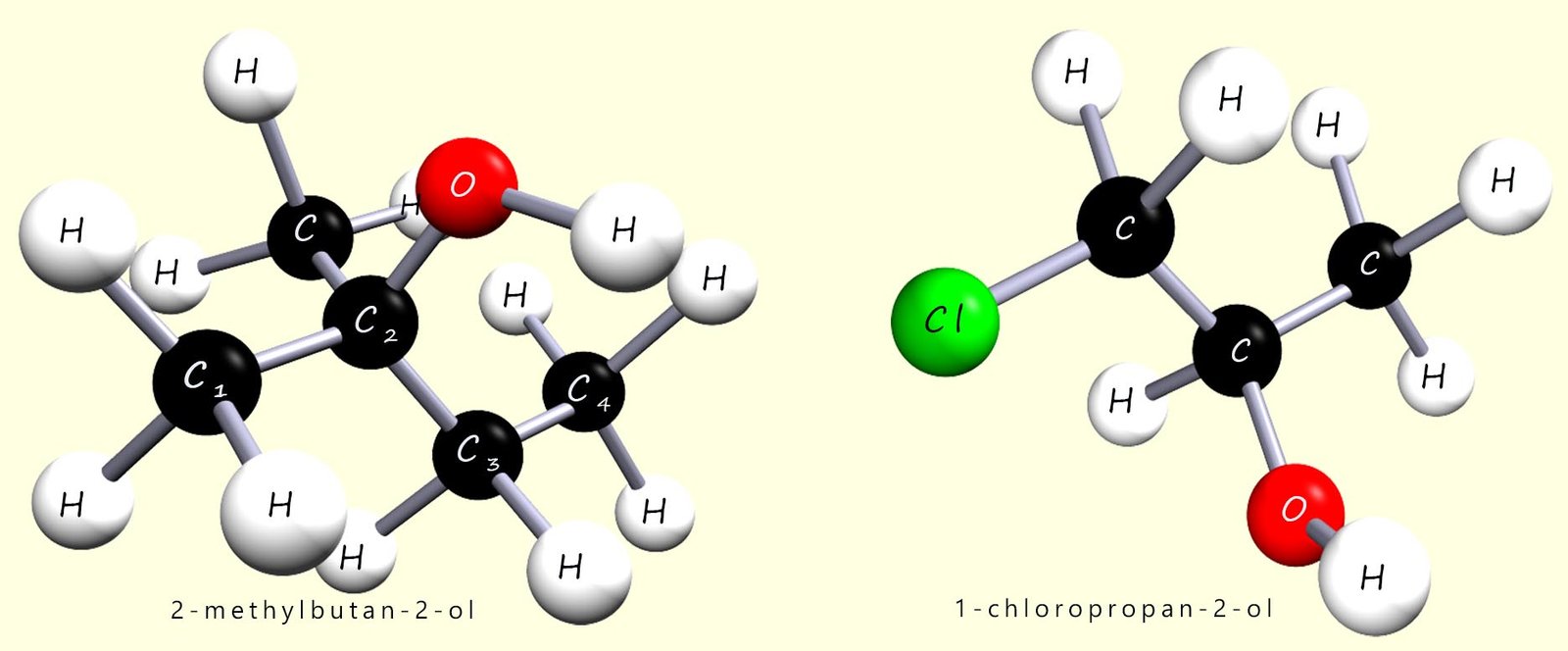
Alcohols are a fairly low-priority functional group, hey are fairly close to the bottom in the ranking table above. If the hydroxyl (–OH) functional group is the highest-priority group present, then the molecule is named as an alcohol and its name simply ends in –ol. However, if a higher-priority functional group is also present, the hydroxy group (–OH) group is treated as a substituent and is identified using the prefix –hydroxy instead. This is explored in more detail later in this page.
For each molecule below, identify the –OH group, choose the longest carbon chain containing –OH, number the chain correctly, then name the molecule.
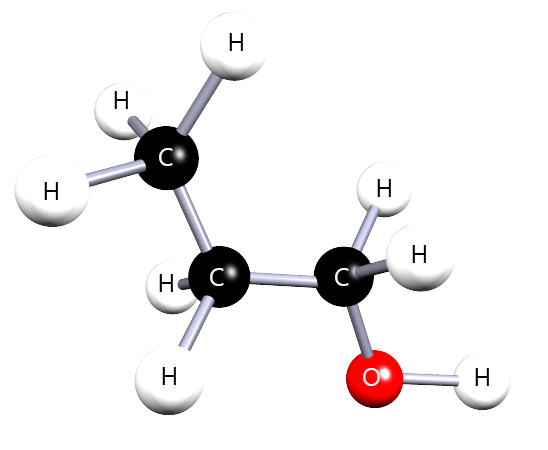
Correct name: propan-1-ol
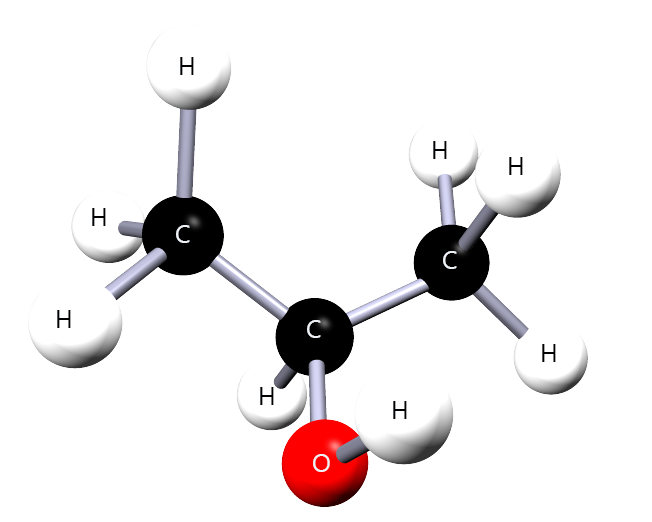
Correct name: propan-2-ol
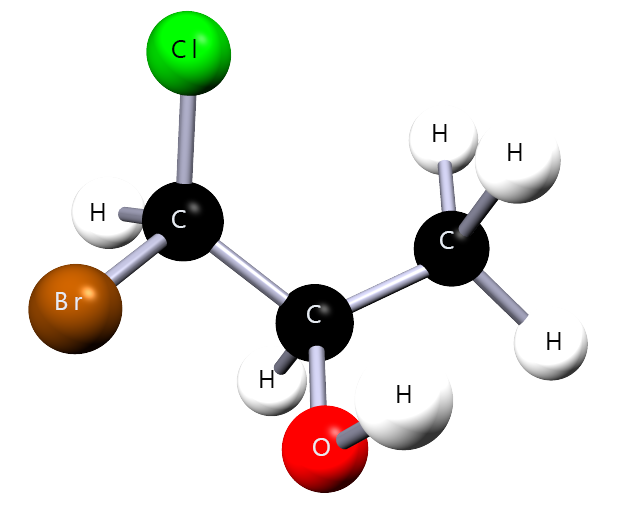
Correct name: 1-bromo-1-chloropropan-2-ol
Carboxylic acids are a family of weak acids which contain the carboxyl functional group (-COOH).
| Homologous series | Name | Functional group | general formula |
|---|---|---|---|
| carboxylic acids | suffix -oic acid | COOH | CnH2n+1 COOH |
The first 3 members of the carboxylic acid homologous series are shown below:
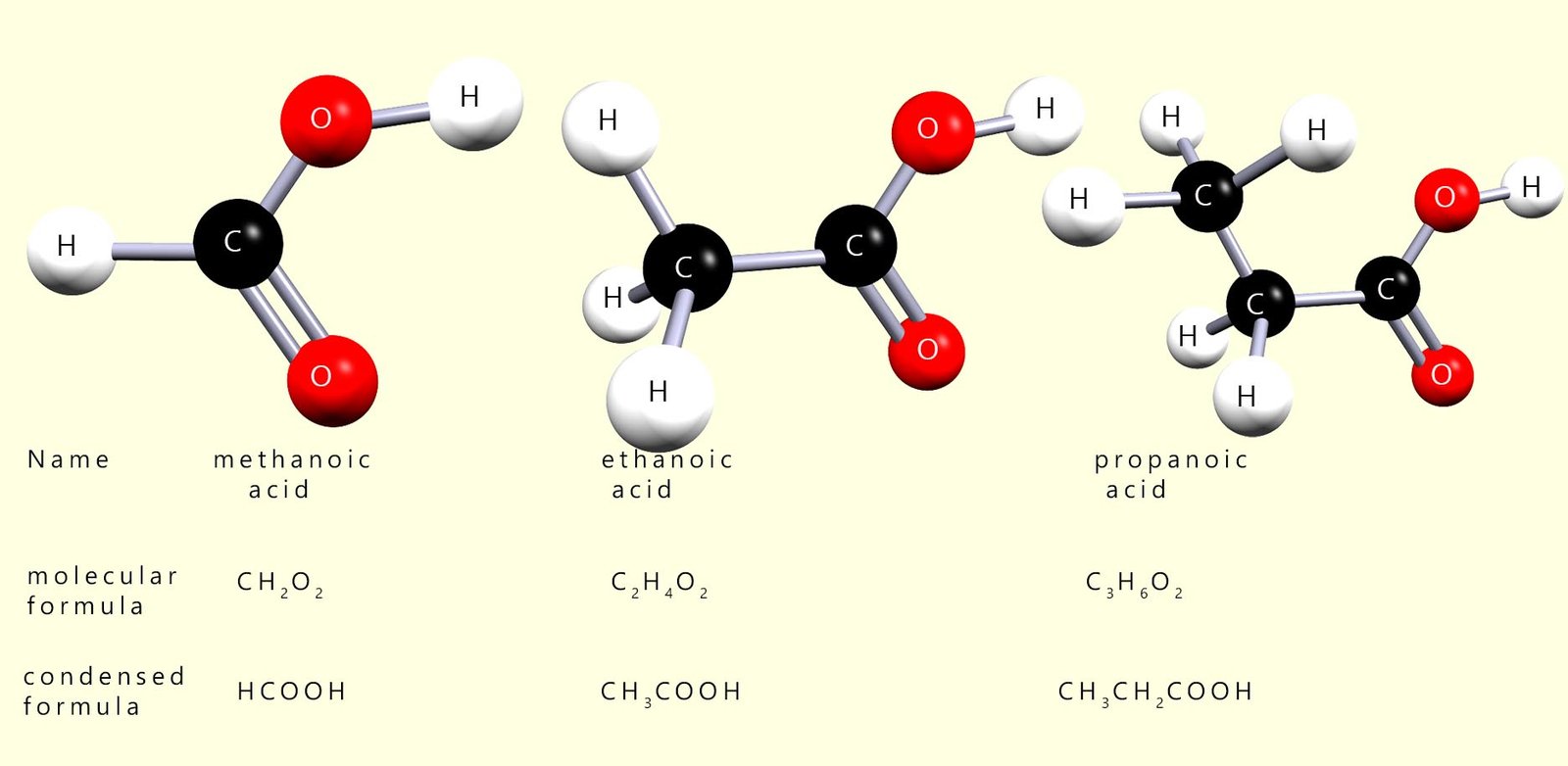
Carboxylic acids all contain the carboxyl functional group (-COOH); they are named as being derived from alkanes. To name a carboxylic acid simply find the longest carbon chain containing the carboxyl functional group and simply replace the -e ending from the corresponding alkane with -oic for the carboxylic acid.
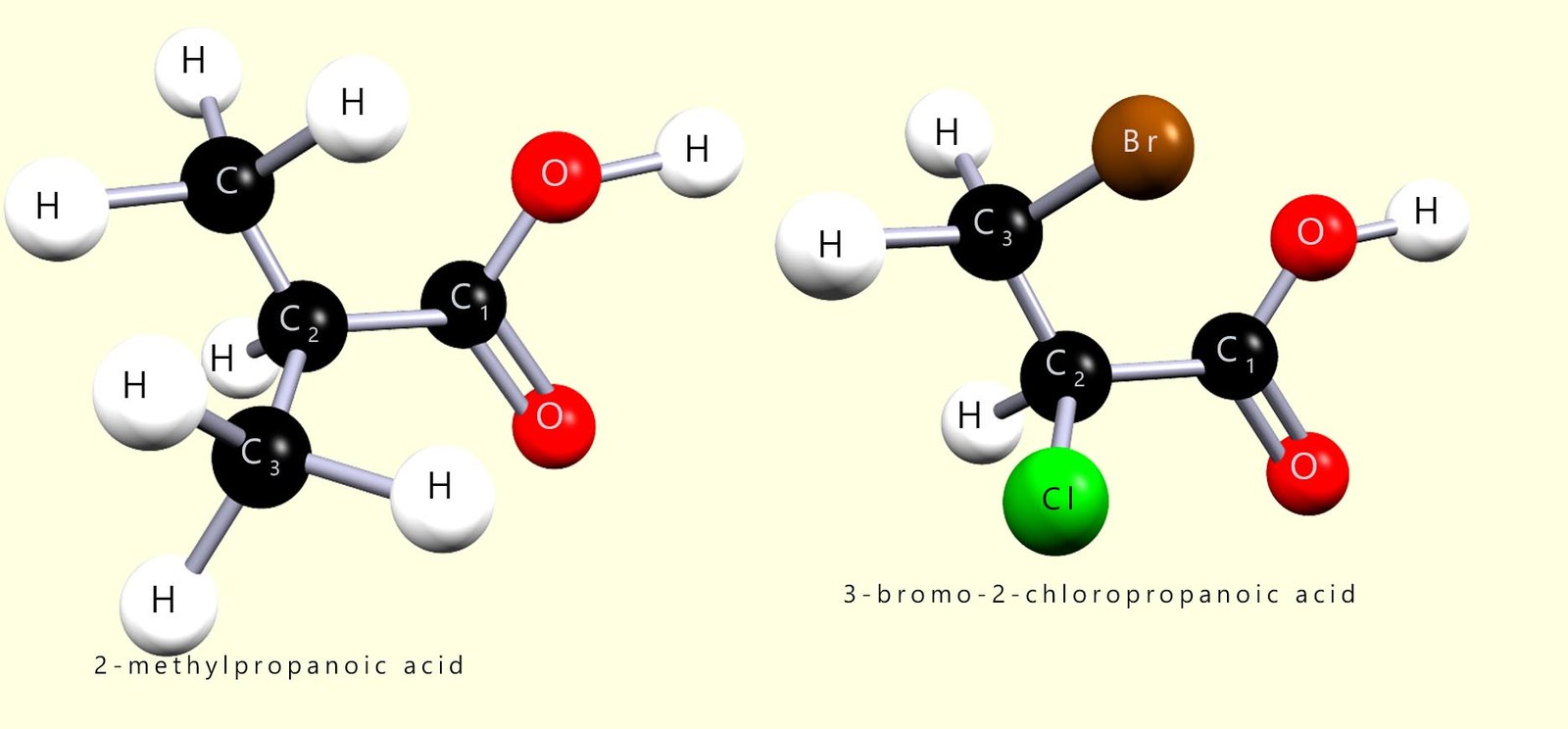
For naming purposes the carboxyl group is a high priority group and the carbon atom in the carboxyl group will always be carbon atom number 1 in the longest carbon chain in the molecule e.g. two examples of substituted carboxylic acid molecules are shown below.
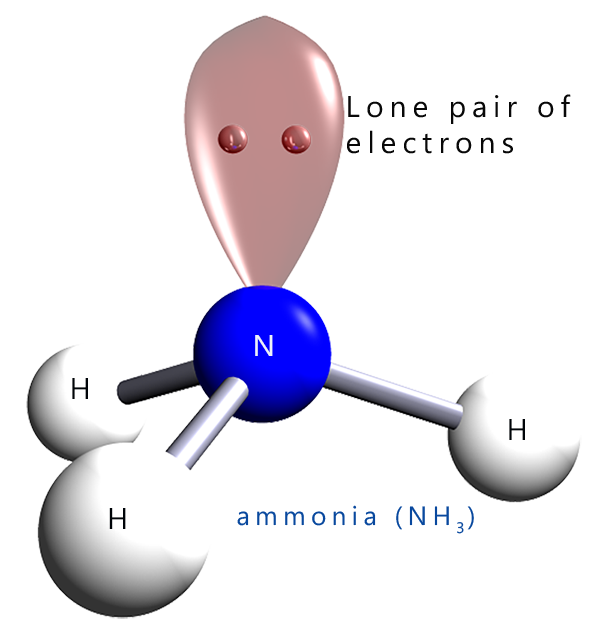
Amines can be thought of as derivatives of ammonia (NH3); shown opposite. Amines are formed by simply replacing one, two or even all three of the hydrogen atoms on a molecule of ammonia with an alkyl group such as a methyl (-CH3), ethyl (-C2H5) or a propyl (-C3H7) groups. Amines are classified as primary, secondary or tertiary amines depending on how many of the hydrogen atoms in an ammonia molecule have been replaced. In a primary amine one hydrogen atom in an ammonia molecule has been replaced by an alkyl group; while in a secondary amine two hydrogen atoms have been replaced by alkyl groups while in a tertiary amine all three hydrogen atoms in an ammonia molecule have been replaced by alkyl groups.
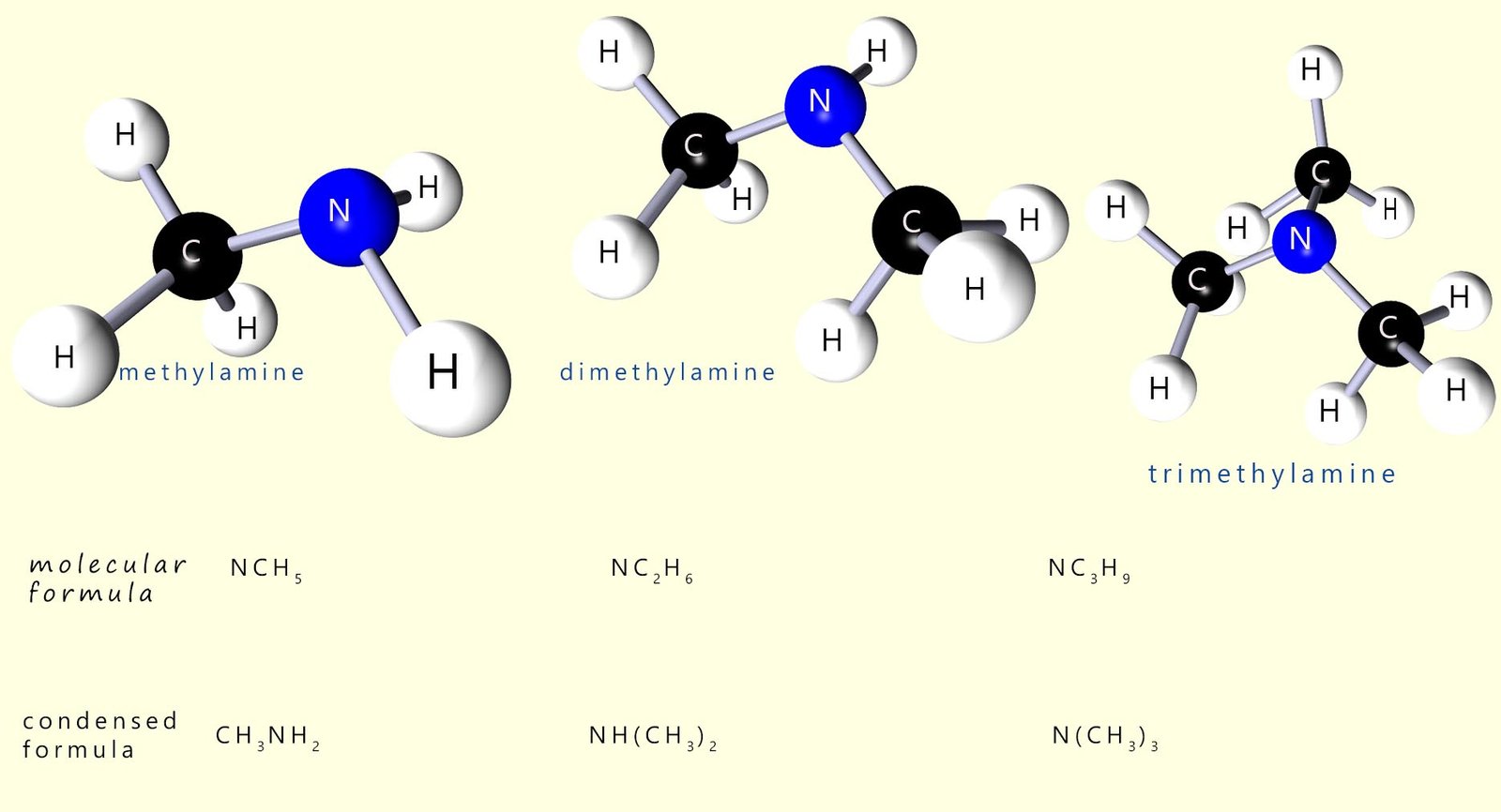
Naming amines can be a bit of a nightmare at times as there are several naming systems in use but luckily most exam boards seem to prefer the traditional naming system I have outlined below. Simple amine molecules are named by simply named by adding the suffix -amine to the name of the alkyl or aryl group; for example the molecules below can be thought of as simply an ammonia molecule where one or more of the hydrogen atoms has been replaced by an alkyl group.
If more than one alkyl group is attached to the nitrogen atom in an amine molecule then the largest alkyl group is chosen as the parent name and the other alkyl group or groups are assigned as a N-alkyl group, for example in each of the molecules shown below the largest alkyl chain is a propyl chain (C3H7), so this will be the parent name of the amine. The other substituent(s) are labelled as N-alkyl substituents to indicate that they are attached to the nitrogen atom:
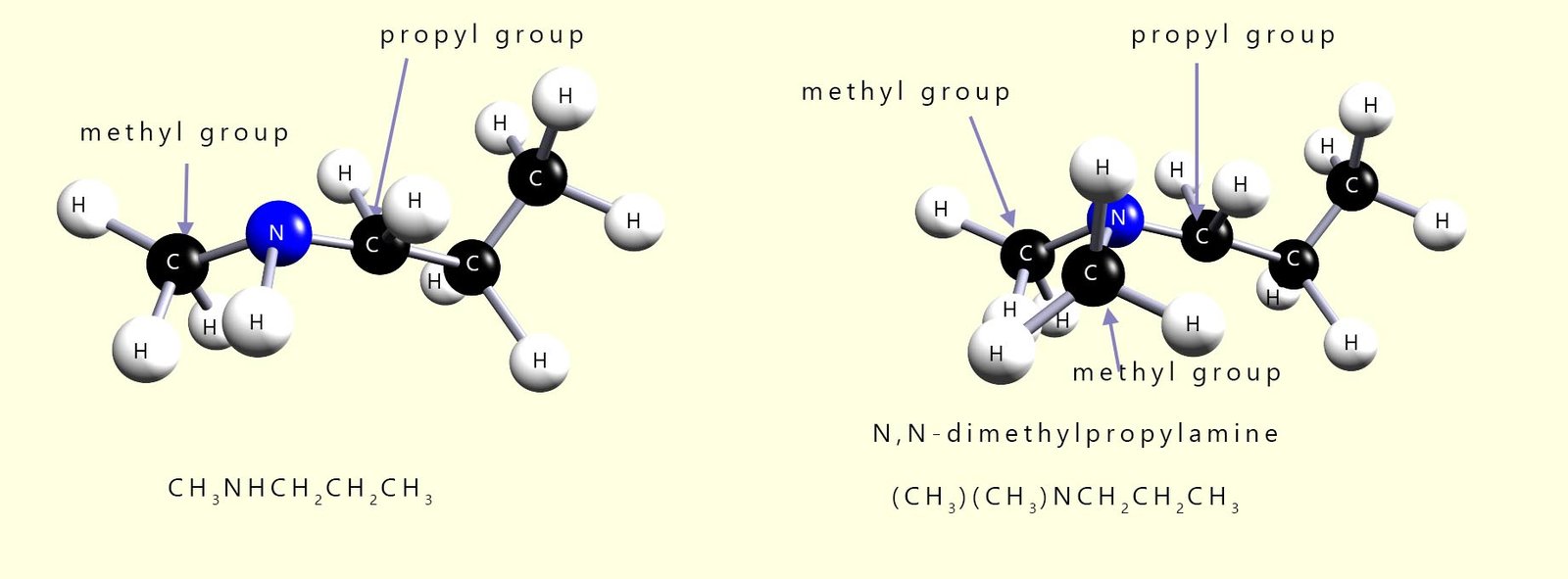
You may come across another method used to name amines whereby instead of using the suffix -amine to name the compounds instead the prefix -amino is used; the image below gives several examples of this naming system. However most exam boards seem to prefer the traditional naming system above so I would use this unless your teacher tells you otherwise.
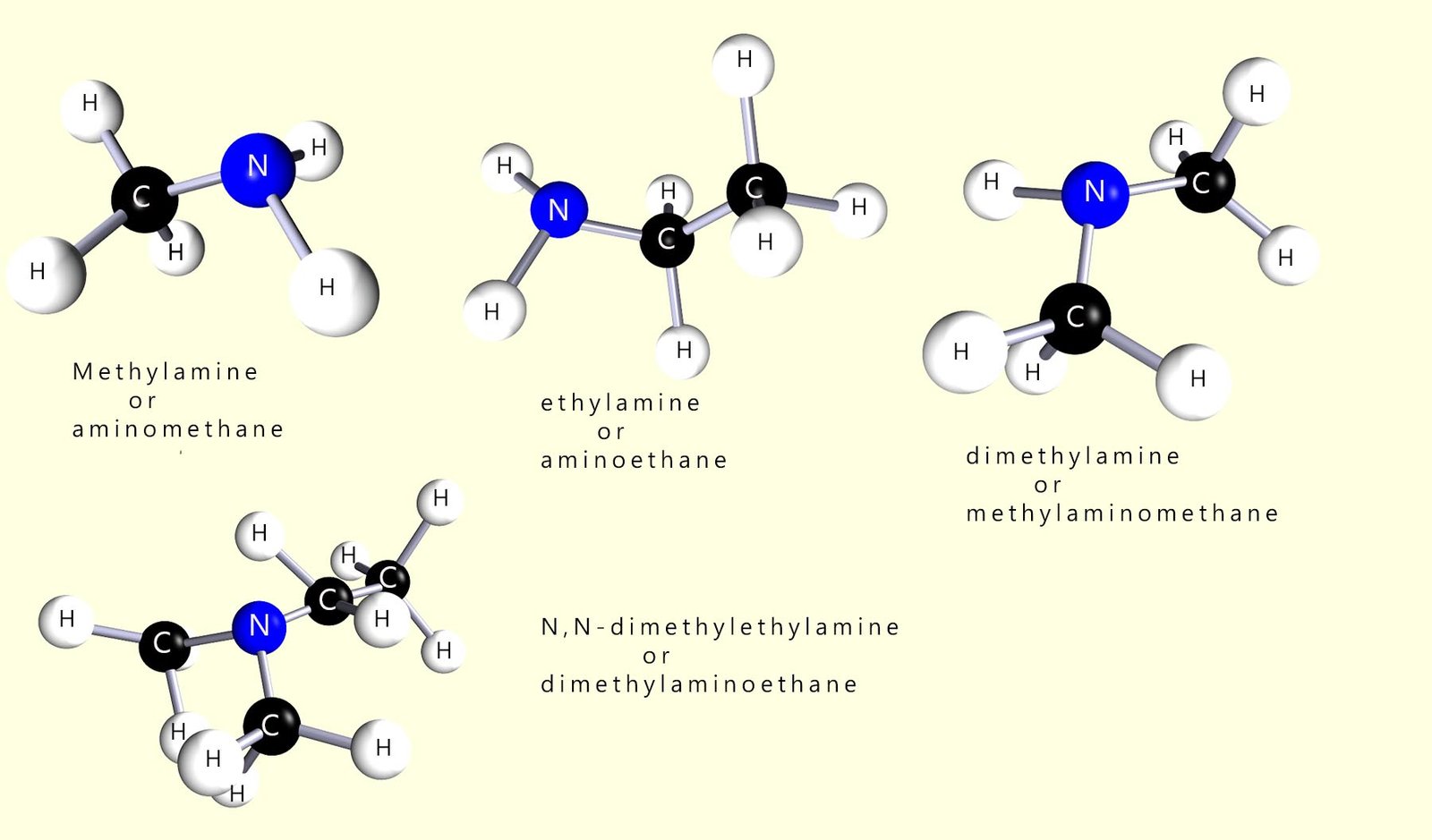
So for example simply use the prefix amino in front of the name of the longest alkyl chain present in the molecules below. The first two examples are primary amines while the third example is a secondary amine. Here use the same rule, identify the longest alkyl chain which will be the parent name and other substituents will be labelled as N-substituents.
Use the rules above to name the amine shown below. Work through the steps before revealing the answer.
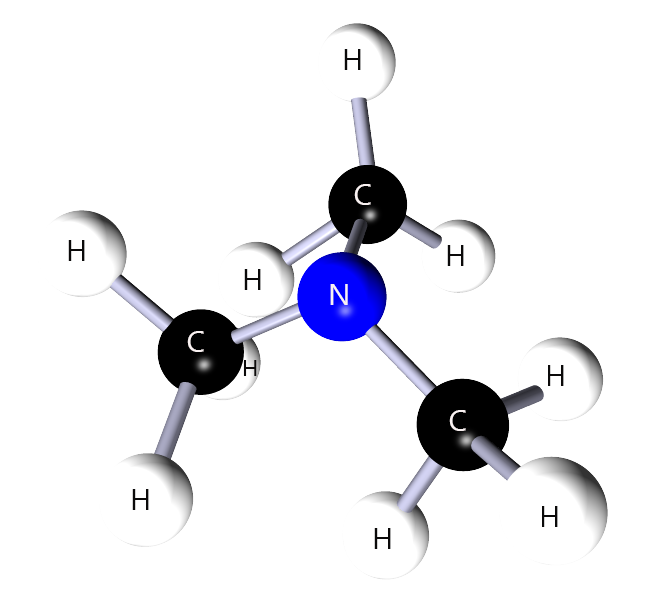
Correct name: trimethylamine or dimethylaminomethane
Aldehydes are another family of organic molecules that contain the functional group (-RCHO). This consists of a carbonyl group (-C=O) bonded to an R (alkyl or aryl) group and a hydrogen atom. The -RCHO carbon is always numbered as carbon atom number 1 in the carbon chain in an aldehyde molecule.
| Homologous series | Name | Functional group | general formula |
|---|---|---|---|
| aldehydes | suffix -al | RCHO | CnH2nO |
The first 3 members of the aldehyde homologous series are shown below:
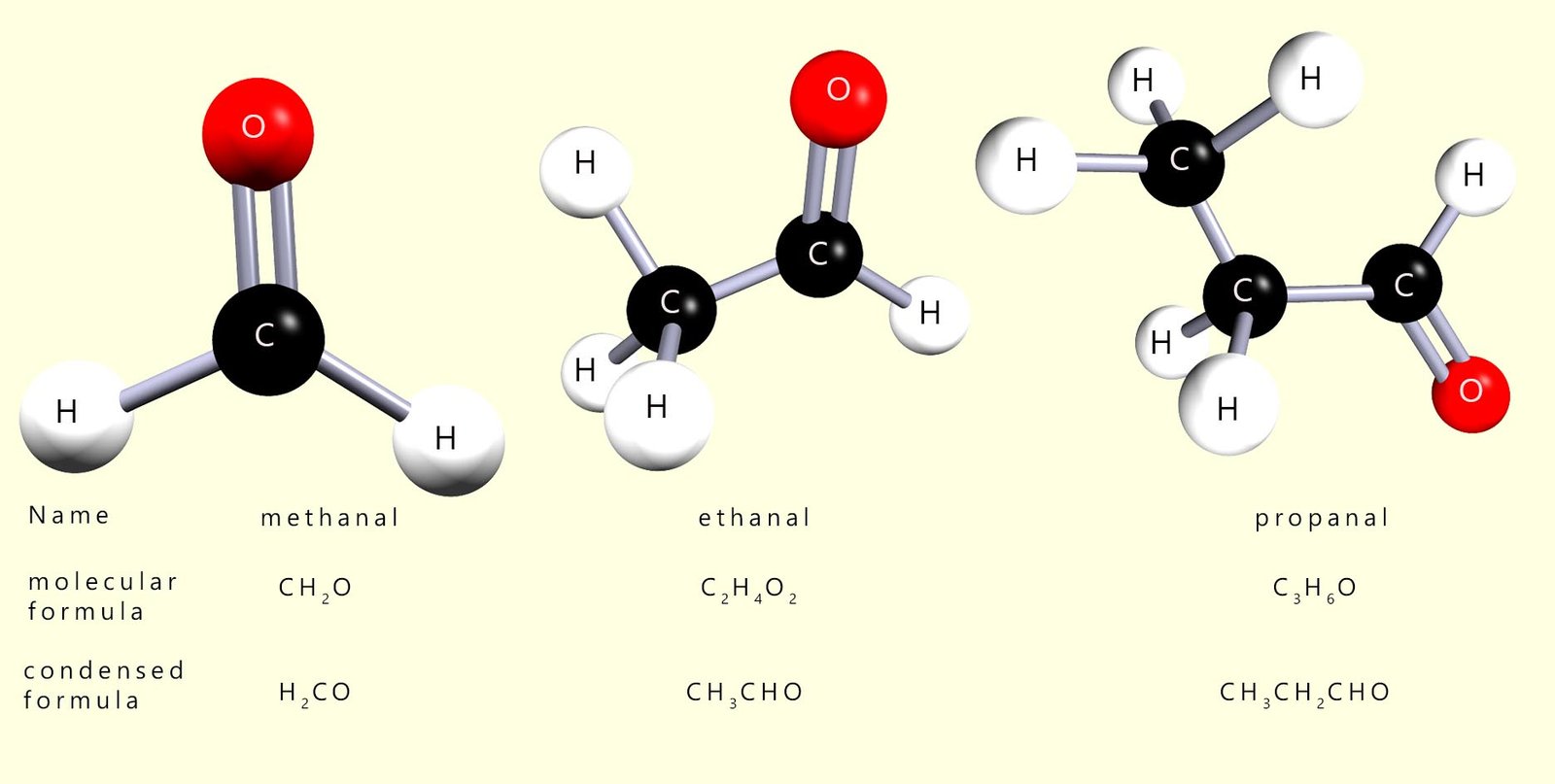
Aldehydes are named by simply replacing the -e on the longest alkyl chain that contains the CHO functional group with the suffix -al. The carbon atom in the CHO functional group is always numbered as carbon atom number 1 when we come to naming aldehydes e.g. study the example below which shows a substituted aldehyde molecule.
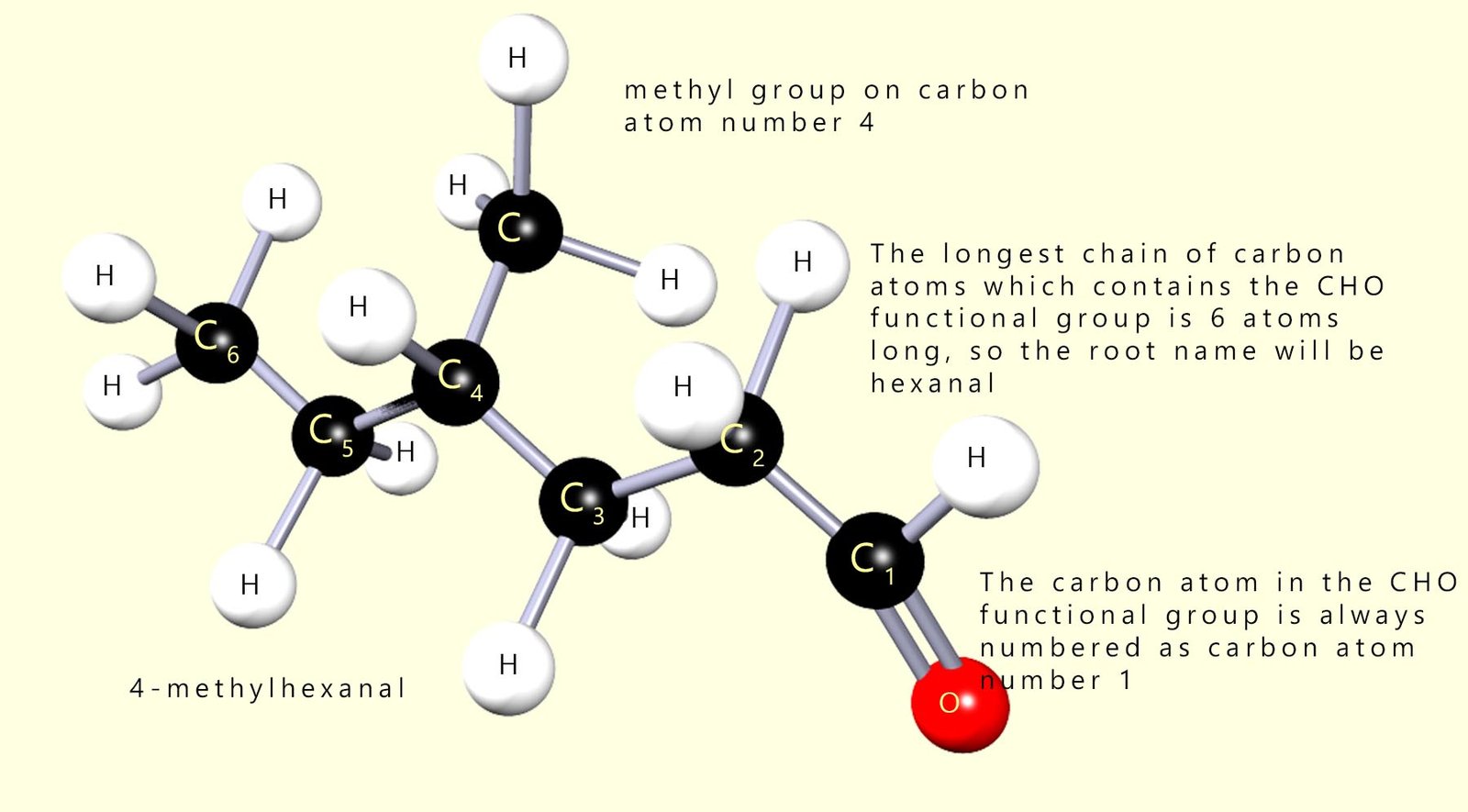
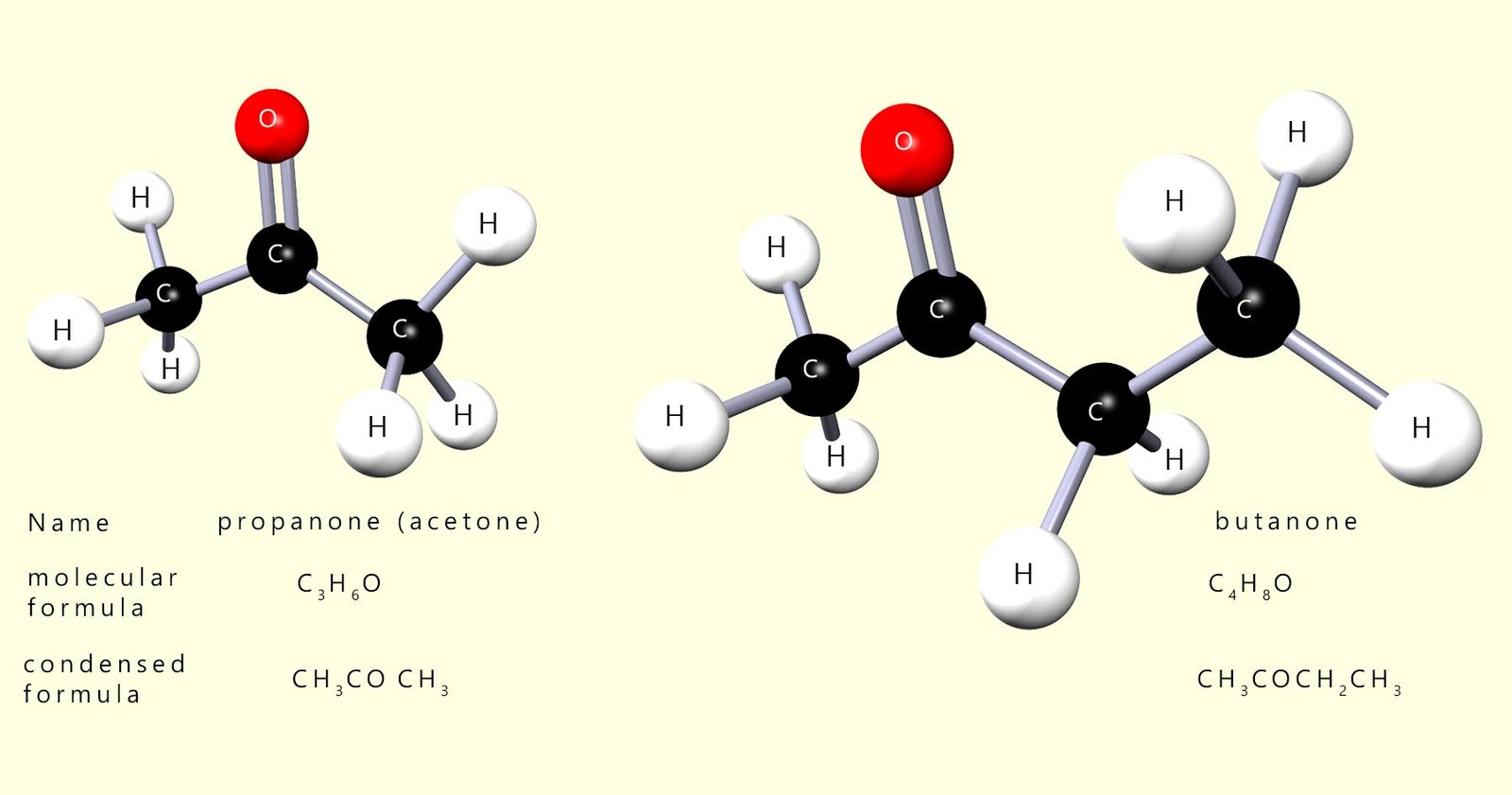
Ketones contain the functional group R2CO (RCOR) and they are named by replacing the -e of the corresponding alkane with -one. The longest carbon chain selected in any molecule to be named as a ketone must contain the ketone functional group. Ketones are named in such a way as to ensure that the carbonyl carbon has the lowest possible number e.g.
| Homologous series | Name | Functional group | general formula |
|---|---|---|---|
| ketone | suffix -one, prefix -oxo |
RCOR | CnH2nO |
Ketones have a structure which is similar in many ways to that found in aldehydes, the only difference is that the carbonyl carbon is bonded to two alkyl groups in a ketone whereas in an aldehyde it is bonded to an alkyl group and a hydrogen atom. The first 2 members of the ketone homologous series are shown below:
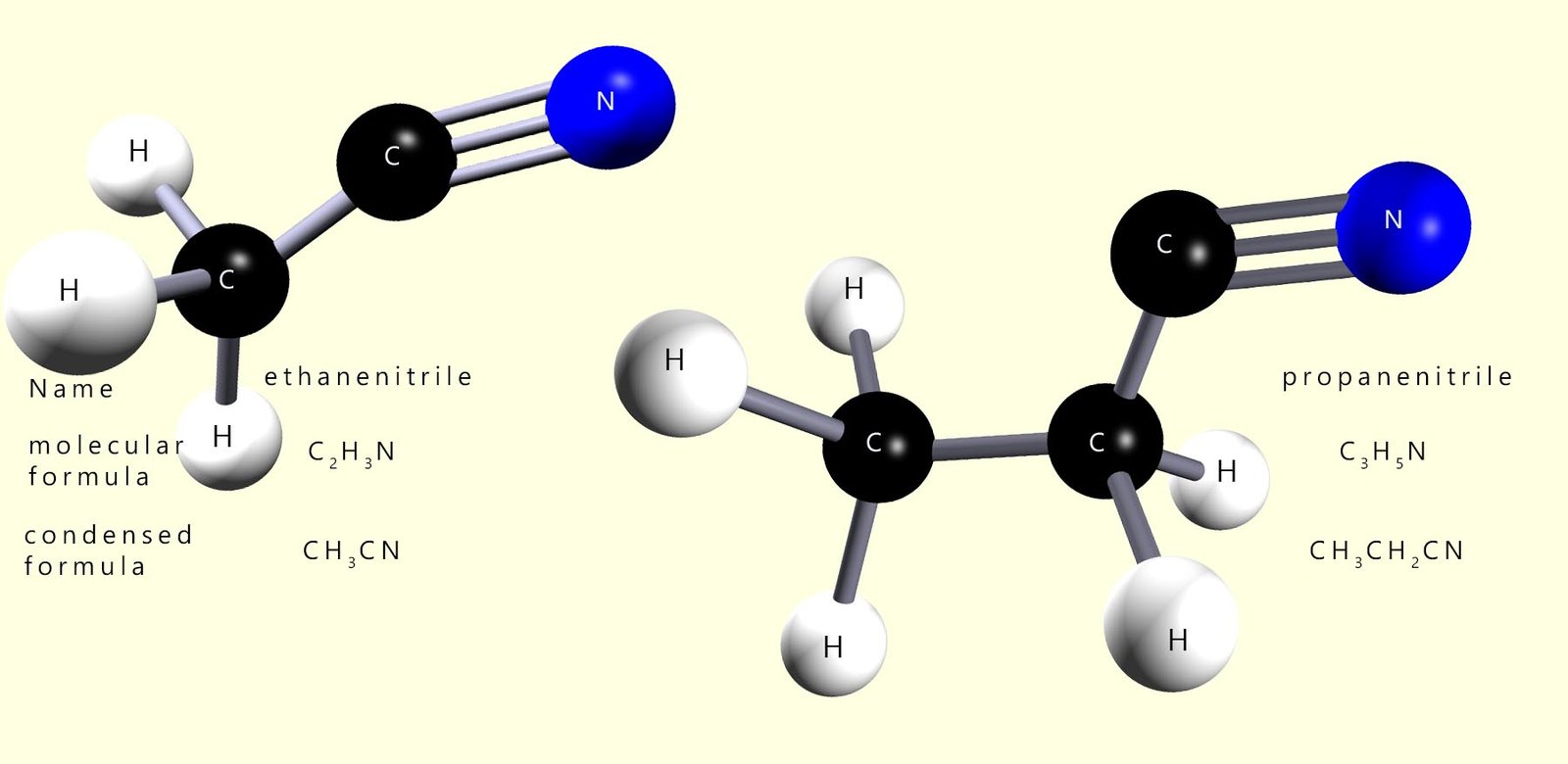
Nitriles contain the functional group R-CN. Simple nitriles are named by adding the suffix -nitrile to the alkane root name. If the molecule containing the nitrile functional group also contains a functional group of higher priority then the prefix cyano is used to indicate the presence of the nitrile group.
| Homologous series | Name | Functional group |
|---|---|---|
| nitrile | suffix -nitrile prefix - cyano |
RCN |
The image below shows 2 simple nitrile molecules.
Ethers contain the functional group R-O-R; where R is an alkyl group. To name a simple ether identify the longest carbon chain in any of the attached alkyl groups and use this as the parent alkane. The smaller alkyl group with the shorter carbon chain attached to the oxygen atom is then named as an alkoxy substituent for example:
The alkoxy group is written before the name of the parent alkane, so for example an ether with a methoxy group attached to an ethane chain would be named methoxyethane; this is shown in the image below:
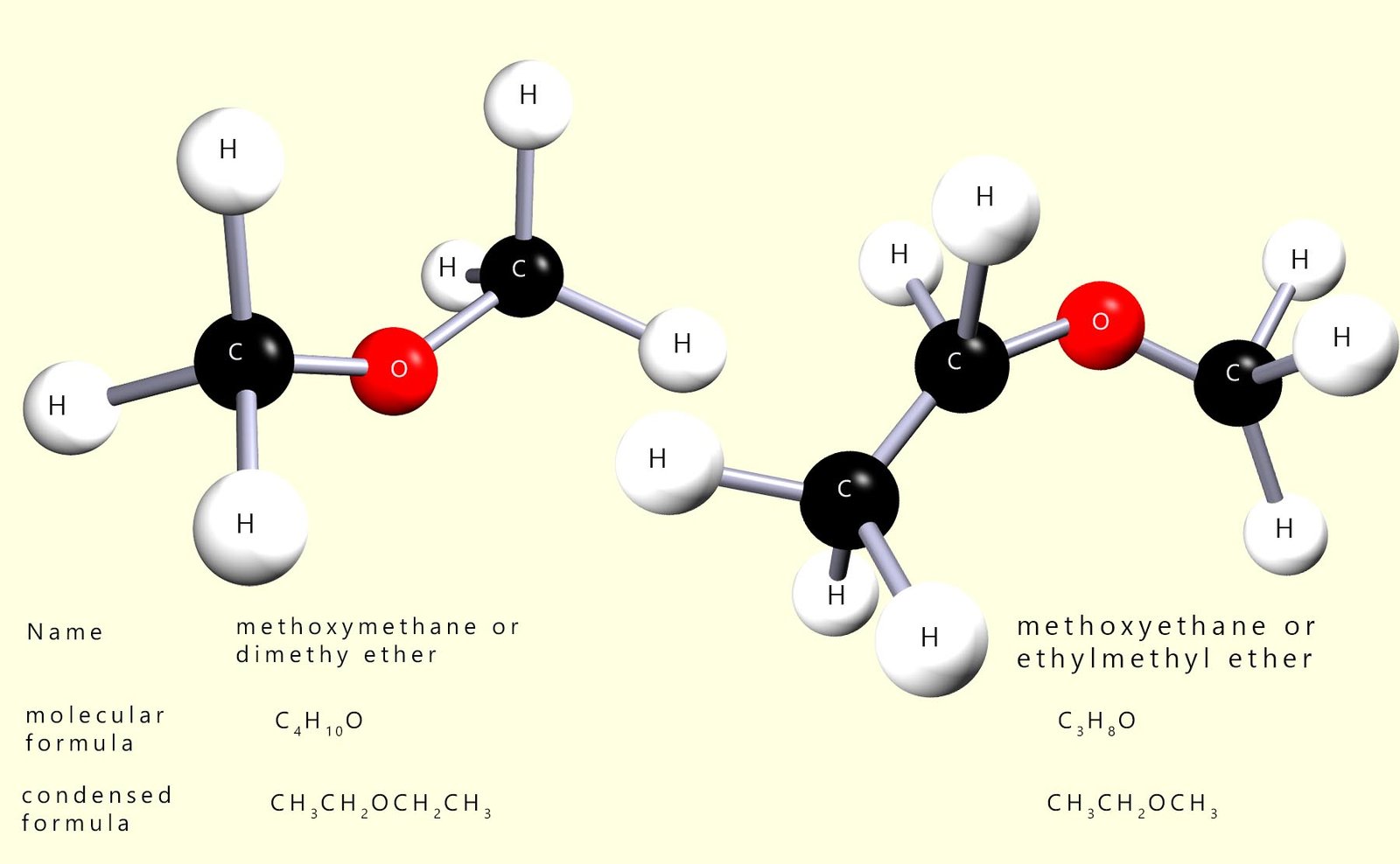
An alkoxy substituent is simply a carbon chain that is attached to an oxygen atom. It is named in the same way as an alkyl group, but with the ending -yl replaced by -oxy. For example a methyl group (-CH3) becomes a methoxy group (-OCH3, and an ethyl group (-C2H5) becomes an ethoxy group (-OC2H5. When naming an ether, the alkoxy group is simply treated as a substituent on the longer carbon chain and is written before the name of the parent alkane.
You may also see simple ethers named using the older, common naming system where the two alkyl groups are listed alphabetically followed by the word ether, for example methylethyl ether. However the systematic name methoxyethane is the one you should use in your exams.
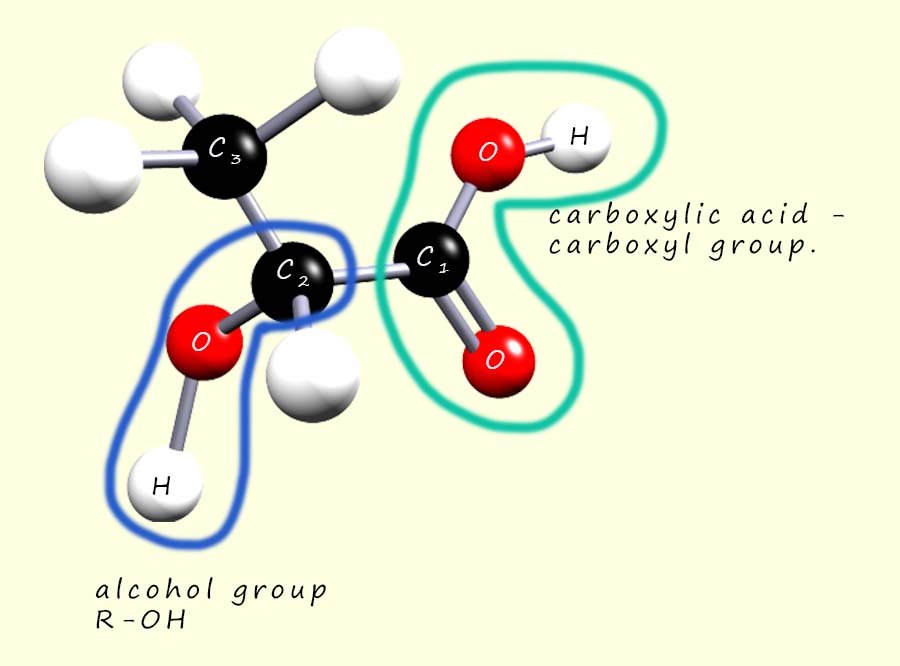
Consider the molecule shown opposite. This molecule has
| Order of priority |
|---|
| carboxylic acid |
| ester |
| nitrile |
| aldehyde |
| ketone |
| alcohol |
| amine |
| alkene |
| halogen |
So how do we name this molecule? Is it named as a carboxylic acid
or as an alcohol? To name it properly we just
follow some simple rules. The functional groups present in a
molecule can be ranked in order of priority for naming purposes; we saw this earlier when naming substituted d halogenalkane molecules. For
example carboxylic acids take precedence over nitriles which take precedence over aldehydes and ketones which
take precedence over alcohols which take precedence over amines, the table opposite lists the order of priority when it comes to ranking functional groups.
So in this particular molecule the carboxylic acid functional group will take priority over the alcohol hydroxyl functional group, this means that
the molecule will be named
as a carboxylic acid with an alcohol
substituent attached.
Recall that in a carboxylic acid the carbon atom present in the carboxyl functional group is numbered as carbon atom number 1. So in this molecule the alcohol hydroxyl group (-OH) is attached to carbon atom number 2. Now the longest chain of carbon atoms containing the carboxylic acid functional group is 3 carbon atoms long, so the carboxylic acid will be propanoic acid with the alcohol substituent on carbon number 2.
Now the prefix -hydroxy is used to identify the alcohol group (R-OH) if it is named as a low priority group in a molecule (see the section above on alcohols). If the molecule was to be named as an alcohol then we would use the suffix -ol; however when it is present in a molecule with a higher priority ranking group then we use the prefix -hydroxy. So this molecule will be named as 2-hydroxypropanoic acid.
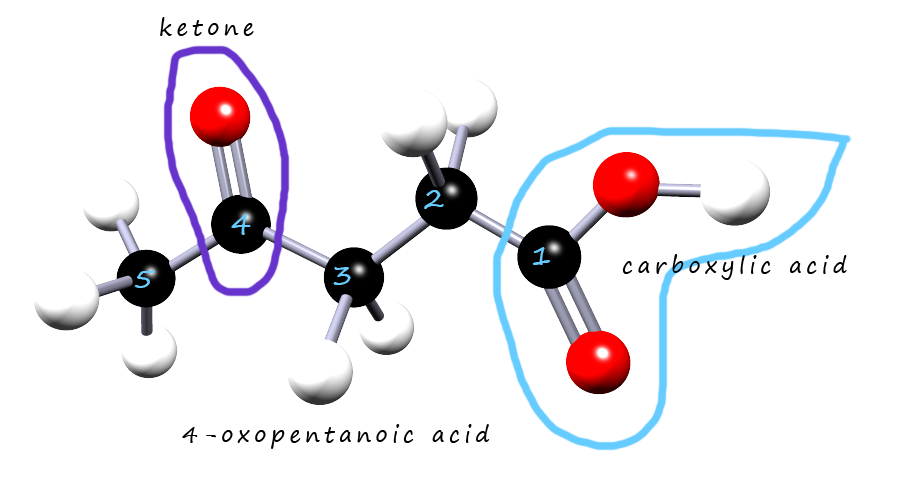
This molecule has two functional groups present:
The carbonyl group (ketone) will be treated as a substituent on carbon atom number 4. The prefix oxo is used to identify a ketone group with low priority. So the molecule will be called 4-oxopentanoic acid.
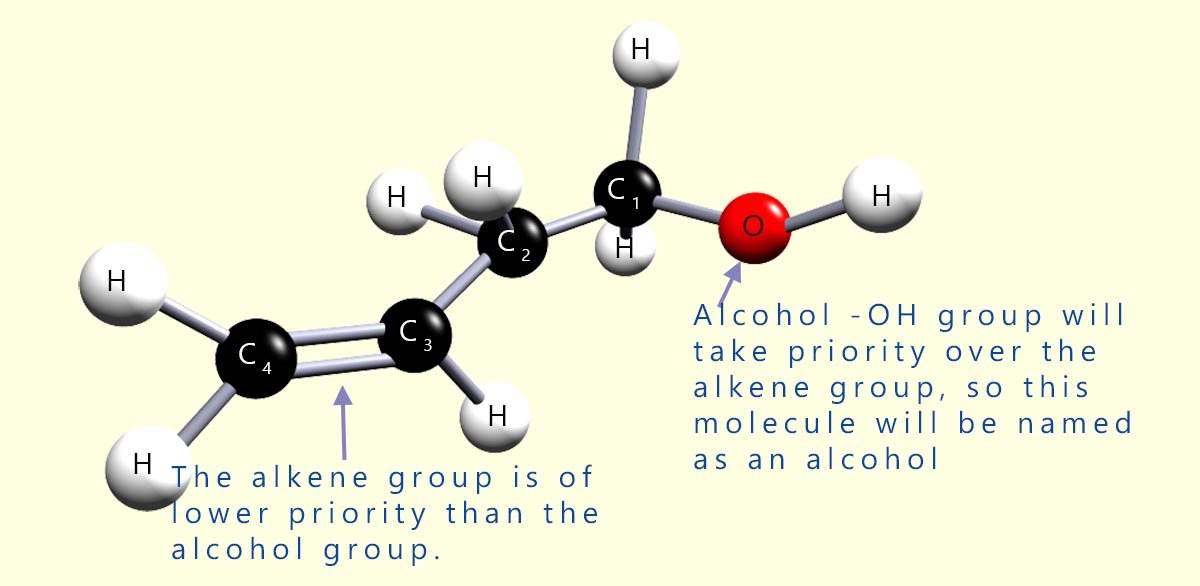
The molecule shown opposite contains two functional groups; an alcohol hydroxyl group and an alkene (C=C) unsaturated group. From the table of priority above we can see that the alkene group is a low priority group.
In the molecule shown opposite then the alcohol functional group, the hydroxyl group (-OH) will take priority over the alkene group; this means that the molecule will be named as an alcohol using the suffix -ol. The location of the C=C group is noted by listing the carbon atom where the double bond starts, and using the infix -en- in the name. In this case the double bond starts at carbon atom number 3. The longest carbon chain in the molecule is four carbon atoms so the molecule will be named as a derivative of butan-1-ol. The molecule opposite will be called but-3-en-1-ol
| Functional group | Group formula | Suffix (highest priority) | Prefix (lower priority) |
|---|---|---|---|
| Carboxylic acid | –COOH | –oic acid | carboxy– |
| Ester | –COO– | –oate | alkoxycarbonyl– |
| Nitrile | –C≡N | –nitrile | cyano– |
| Aldehyde | –CHO | –al | formyl– |
| Ketone | >C=O | –one | oxo– |
| Alcohol | –OH | –ol | hydroxy– |
| Amine | –NH2 / –NR2 | –amine | amino– |
| Alkene | C=C | –ene | en– |
| Halogen | –X | — | fluoro–, chloro–, bromo–, iodo– |
| Ether | –O– | — | alkoxy– |
🧠 Exam reminder: only one functional group gets a suffix — all other groups must be named using prefixes.