Fractional distillation of crude oil
Crude oil, despite being one of the most valuable natural resources, is a useless, smelly and thick
black "liquid" when it comes out of the ground. The reason for this is that it is a mixture. It is a
mixture of compounds called hydrocarbons.
Hydrocarbons are compounds containing only the elements
hydrogen and carbon. There are thousands of different hydrocarbons
all mixed together in crude oil.
Some of the hydrocarbon molecules are small, some are medium sized and some are large. At an oil
refinery the mixture of hydrocarbons present in the crude oil is separated into different fractions of hydrocarbons which contain similar sized molecules; this is easily
done since similar sized molecules have similar boiling points.
The image below shows what happens to the crude oil
at an oil refinery and explains how it is separated into its various fractions.
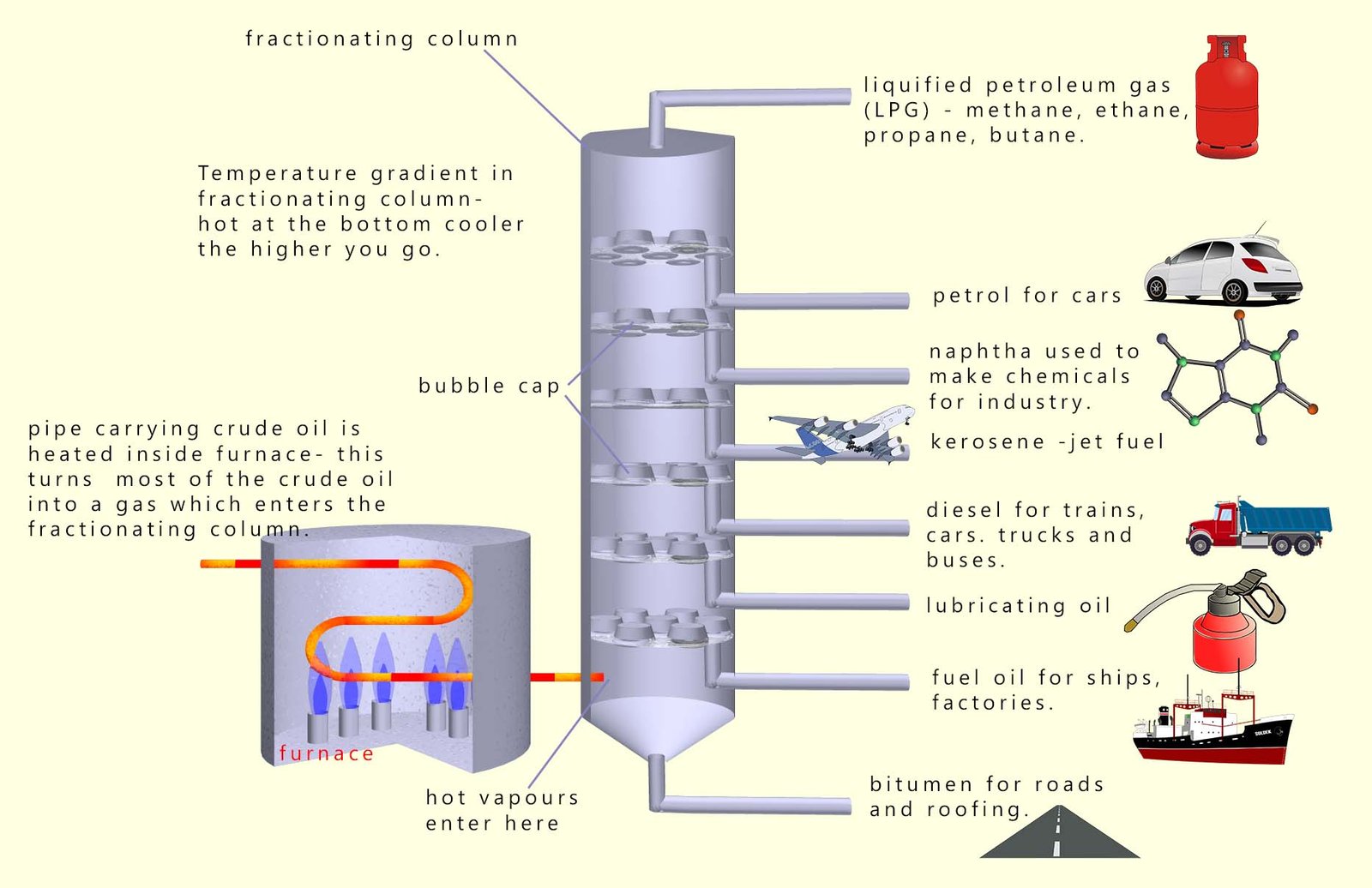
At the oil refinery:
- The crude oil passes through a hot pipe in a furnace, this vaporises most of the hydrocarbon molecules present in the crude oil.
- The oil vapours then enter the fractionating column. This is a tall narrow cylinder with a temperature gradient;
it gets cooler the higher you go up the column.
- The hot vapours rise up inside the fractionating column. When the hot vapours meet a surface at a temperature cooler
than their boiling point then they will condense. This is why, for example, if you boil a kettle too close to
a cool window condensation collects on the window. The steam from the kettle, which may be around 100°C, meets the window which is cooler than the boiling point of the water; so the steam will condense. The same process
will happen inside the fractionating column. Hot vapours will rise until they meet a bubble cap lower
than their boiling point, the hot vapours will then condense and the liquids formed will be run off and
collected.
- Any fraction or part of the oil that was not vaporised will fall to the bottom of the column and is collected. This will be the fraction containing bitumen (tar) and greases.
- The larger or longer the hydrocarbon molecule the higher
will be its boiling point. The fractionating column will basically separate out molecules by their boiling points into separate fractions. Each fraction will
contain many different hydrocarbon molecules each with similar boiling points.
As you can see lots of very valuable substances such as fuels, petrol, diesel, kerosene for modern
transport, oil to lubricate engines and machinery as well as feedstock molecules in the naphtha
fraction which may end up being used in medicines, cosmetics, solvents, detergents, plastics, as well
as many other everyday products.
- Each fraction collected will contain a mixture of many different molecules. These molecules will be of a similar size with similar boiling points.
Self-check
Try the quick activity below to review your understanding of fractional distillation:
Self-check: Quick Quiz
Try the quick quiz below to review your understanding of fractional distillation:
Key Points
⚠️ Common misconceptions
- ❌ Fractions are pure substances
- ❌ Small molecules condense first
- ❌ All molecules in a fraction are identical
- ❌ Crude oil is a single compound
- Crude oil is a mixture of many different sized hydrocarbon molecules.
- Crude oil is separated out into different fractions at an oil refinery. Here the crude oil is heated until most
of it vaporises. The hot vapours then enter a tall fractionating column where they condense at different heights inside the column.
- The fractionating column has a temperature gradient; it is hot at the bottom
and cooler at the top.
- Small hydrocarbon molecules have low boiling points; large hydrocarbon molecules have high boiling points. Inside the
fractionating column large hydrocarbon molecules condense at the bottom and as you rise up the column the smaller molecules condense higher up.
Practice questions
Next


