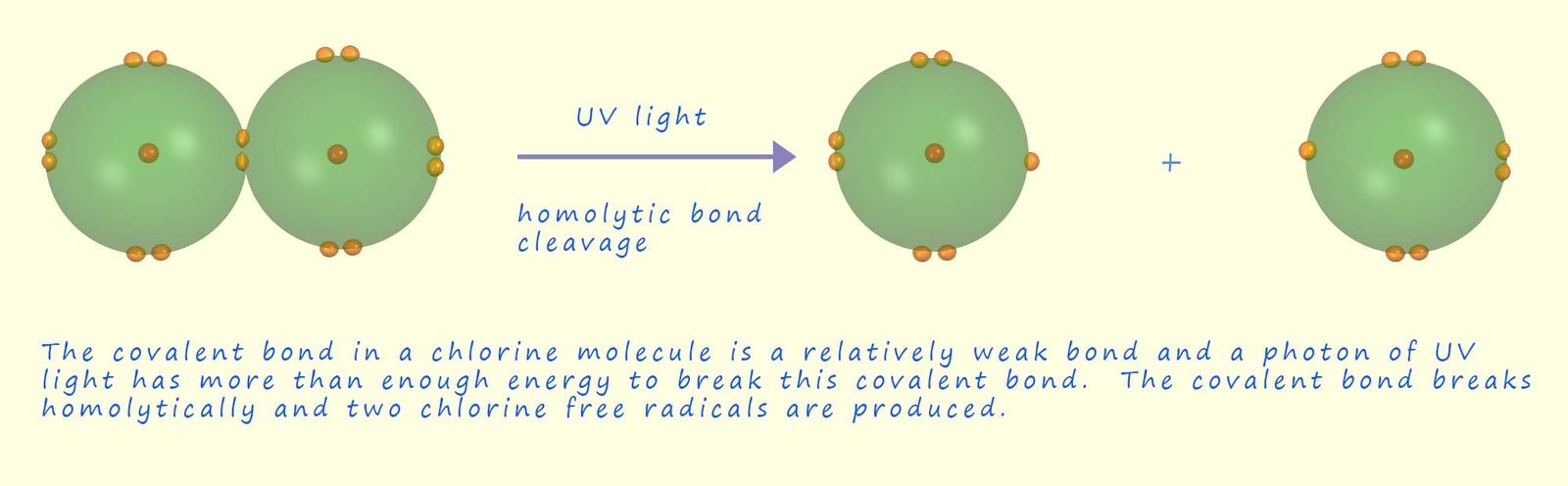
Ever wondered how methane can suddenly react with chlorine when it normally does nothing at all? The answer lies in ultraviolet light and some extremely reactive particles called free radicals. Ultraviolet light (UV) has more than enough energy to break the covalent bonds holding some molecules together; the covalent bonds will break in a homolytic manner resulting in the formation of atoms with an unpaired electron.
These atoms with unpaired electrons are called free radicals and they are very reactive. The image below shows how the covalent bond between the two atoms in a chlorine molecule (Cl2) can be broken homolytically by UV light to form two chlorine free radicals.
The bond enthalpy of the
chlorine covalent bond in a chlorine molecule is only 242kJmol-1. This
means that a photon of UV light has more than enough
energy
to break or cleave the Cl-Cl covalent bond and form two chlorine free radicals; as shown in the diagram above. The two equations shown below also show this homolytic bond cleavage taking place. Since each chlorine atom has an identical electronegativity value when the bond breaks each chlorine atom is able to attract its own electron and form the two chlorine free radical containing an unpaired electron.

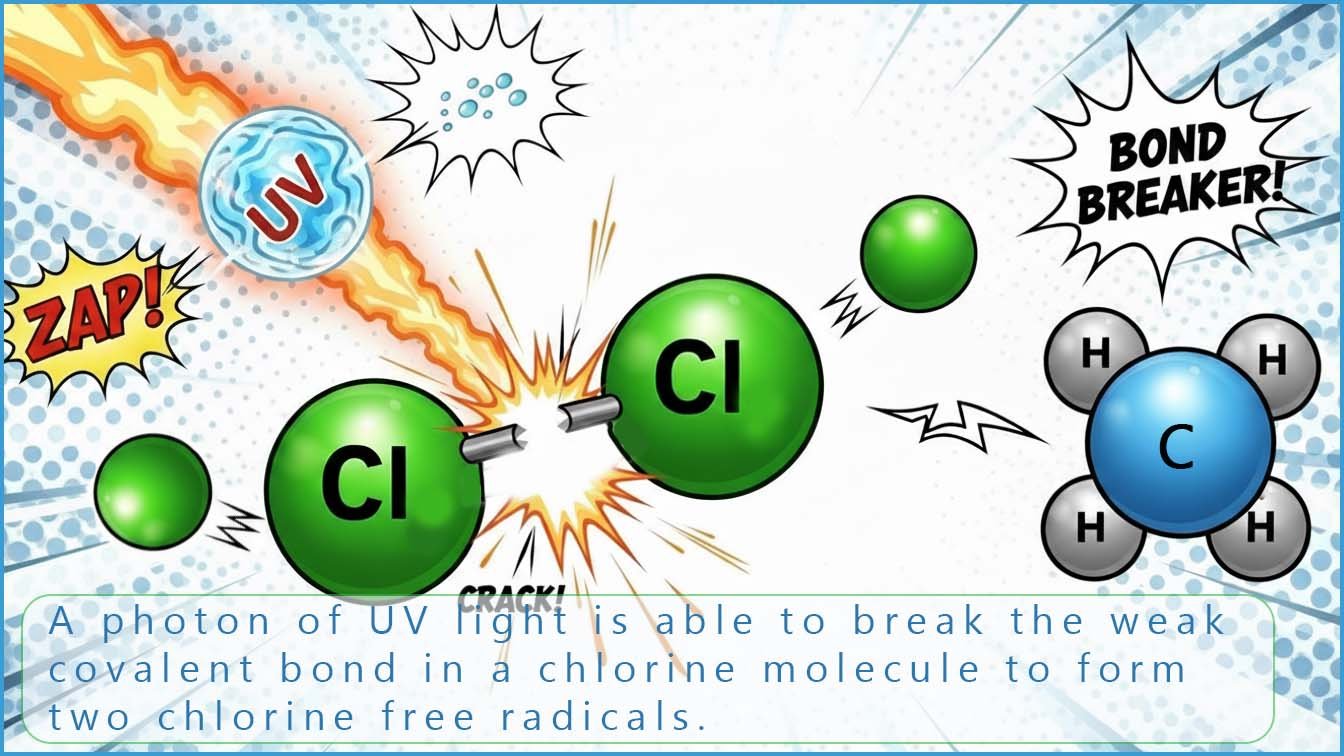
Alkanes are chemically unreactive substances largely due to the fact that the C-H bond in an alkane molecule is non-polar and so is not susceptible to attack by electrophiles or nucleophiles. However alkanes will react violently with chlorine in the presence of sunlight or a camera flash, which is used to start or initiate the reaction. The sunlight contains UV radiation which is the same type of radiation responsible for sunburn and over exposure to UV radiation can also prematurely age the skin, however for our purposes the ultraviolet radiation will supply enough energy to break the relatively weak bond in a chlorine molecule and form two chlorine free radicals. These free radicals are highly reactive since they contain an unpaired electron and will immediately react with the first "thing" they come into contact with which in our case is likely to be an alkane molecule.
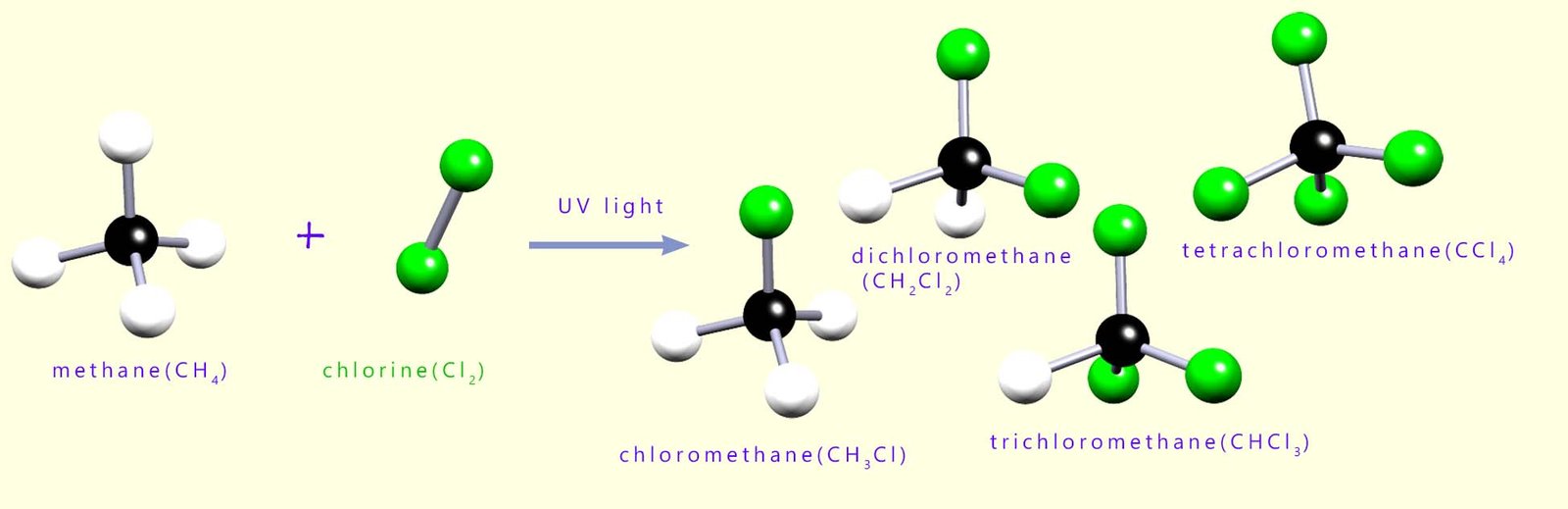
For example chlorine (Cl2) will react with methane (CH4) to form a mixture of chloroalkanes as shown below. The chlorine free radicals produced in this reaction will replace or substitute for the hydrogen atoms on the methane molecule to form a halogenalkane or haloalkane molecule. A mixture of four halogenalkane molecules is likely to be produced in this reaction as each of the four hydrogen atoms in a methane molecule could be replaced by a chlorine atom; these are shown in the image below; but it is possible to adjust the initial reaction mixture to obtain mainly one of these halogenalkane molecules as the main component in the mixture produced.
 This reaction of chlorine with methane
is an example of a free radical substitution reaction, these reactions are free radical chain reactions that proceed
via 3 steps; these 3 separate steps are called:
This reaction of chlorine with methane
is an example of a free radical substitution reaction, these reactions are free radical chain reactions that proceed
via 3 steps; these 3 separate steps are called:
The initiation step
generates or produces the free radicals that are needed to start the reaction between the chlorine molecules and the methane molecules.
It is the presence of a small but constant number of these reactive free radicals
that allows the reaction to proceed.
The free radicals which are needed to maintain this chain reaction are constantly being used up and regenerated while the reaction takes place, the reaction will stop once the concentration of free radicals present drops.
For example if a mixture of methane and chlorine
are placed in a plastic bottle and kept in the dark then no reaction occurs, however if
a bright camera flash is set off then a very violent reaction starts. The camera flash will produce photons of light with
enough energy to break the Cl-Cl bond homolytically to
form 2 chlorine free radicals, we can show this as:
The key to understanding these free radical substitution reactions are the
free radicals. It is the presence of these reactive
free radicals that enables the
chain reaction to start and indeed to continue and produce the product halogenalkane molecules. The two steps that happen in the
propagation steps are shown in the equations below. These reactions are examples of a chain
reaction. This basically means that a chlorine free radical
produced in the initiation step will react with a methane molecule and
this leads to the formation of the product as well as another chlorine
free
radical; which can further react with another reactant molecule,
methane in this case and so
the reaction is essentially one big loop.
The two equations below represent the
propagation steps, both equations start and end with the
formation of a chlorine free radical - the perfect set-up for a recurring loop or chain reaction!
In the propagation stage of this reaction a chlorine
free radical
reacts with
a reactant molecule, the methane (CH4) in this case to form a methyl free radical and hydrogen chloride gas. The methyl
free
radical then goes on to react with the other reactant; chlorine gas to
form the product; chloromethane and a chlorine
free radical is regenerated to carry on the chain reaction. So we started
the propagation step with a
chlorine free radical and we finish
the propagation step with the same chlorine
free radical being produced. The high reactivity
of free radicals and the fact that both the
equations above are exothermic results in this
chain reaction being very violent and
very fast.
Again it is worth highlighting the fact that each step here starts with a free radical reacting with a molecule to form a free radical and a molecule as the product.
These two equations can be combined into an overall equation for the propagation step by simply cancelling out similar species on both sides of the equations as shown below:
Overall equation:
🧠 Exam Brain Box – Free Radical Substitution.
Initiation: UV light causes Cl2 to undergo homolytic bond cleavage, forming two chlorine free radicals.
Propagation: A free radical reacts with a molecule to form a new free radical. Each step starts and ends with a free radical, so the reaction continues as a chain reaction.
Termination: Two free radicals react together to form a stable molecule. No free radicals are produced, so the chain reaction stops.
The reaction is fast and violent because free radicals are very reactive and the propagation steps are exothermic.
The number of free radical present in the reacting mixture at any one time is quite small; however as we have seen in the propagation steps as one free radical is used up another is always produced so the number of free radicals stays fairly constant throughout the reaction. Since the number of free radicals is low the probability of them reacting together is quite low. However when they do combine together two free radicals will form a stable molecule and the chain reaction will come to an end at this point.
To write equations for the termination steps simply react together any two free radicals that are present in the reaction mixture from the propagation steps and the initiation step. In the examples above there are two free radicals present; a chlorine free radical and a methyl free radical so the possible termination steps will simply be:
Two chlorine free radicals can combine to form a molecule of chlorine:
A methyl free radical (CH3.) and a chlorine free radical can combine to form a molecule of chloromethane (CH3Cl):
Another possibility is that two methyl free radicals could combine to form a molecule of ethane:
Try the quiz below to test your understanding of the steps involved in a free radical substitution reaction.
Tap the correct step for each statement. Then press "Check answers".
In the propagation step the overall result is that one of the hydrogen atoms on a methane molecule is replaced by a chlorine atom to form chloromethane. In the first propagation step a chlorine free radical reacts with methane molecule to form a methyl free radical and hydrogen chloride gas. However as the overall reaction proceeds the methane gas being a reactant will be getting used up and the amount present will start to fall; at the same time the amount of chloromethane present from the propagation step will start to increase. This means that ultimately in the first step in the propagation reaction the chloromethane will take the place of methane and this will lead to the formation of dichloromethane:
Overall equation:
❌ Saying the reaction is started by heat. ✅ It must be UV light causing homolytic bond cleavage.
❌ Forgetting the initiation step. ✅ Always show how the first free radicals are formed.
❌ Writing propagation steps that do not regenerate a free radical. ✅ Each propagation step must start and end with a free radical.
❌ Saying free radicals are “cancelled out”. ✅ They are intermediates, not cancelled species.
❌ Claiming only chloromethane is formed. ✅ Further substitution leads to a mixture of products unless excess methane is used.
However you can probably see where this is going! The amount of dichloromethane
present will start to rise and the amount of
chloromethane present will start to fall as the reaction proceeds; so the
dichloromethane will take the place of the
chloromethane in the
first propagation step and trichloromethane
will be formed, remember that every time an alkane molecule goes through the propagation step all that happens is a hydrogen atom on the alkane molecule is replaced by a chlorine atom. So the trichloromethane molecule will then go through the same process as the dichloromethane
and tetrachloromethane will be formed; at this point all the hydrogen atoms
on the methane molecule have
been replaced by chlorine atoms. This means that the final products of this reaction will be a mixture of chloromethane, dichloromethane,
trichloromethane and
tetrachloromethane - not ideal!!
We can however change the reaction conditions to make chloromethane
the main product of the reaction simply by using a large excess of methane. In
the first step of the
propagation reaction the chlorine
free radical will react with the first "thing" it comes into contact with.
So if we add a large
excess of methane then it is likely to come into contact with a
molecule of methane and less likely to meet say a molecule of
chloromethane. By the same argument if we want
to produce tetrachloromethane as the main product then simply use an
excess of chlorine. This
will ensure that all the hydrogen atoms on the methane are replaced by a
chlorine atom.
Use the slider activity below to review your understanding of how changing amounts of reactants present changes the products formed.
Move the slider to see how changing the reactant ratio affects the main product in free radical substitution.
✅ Always state that UV light causes homolytic bond cleavage of Cl2 to form chlorine free radicals (initiation).
🔁 Propagation is a chain reaction: each step starts with a free radical and ends with a free radical (radicals are regenerated).
✂️ Termination: two free radicals combine to form a stable molecule, so no new radicals are produced and the reaction stops.
⚠️ Don’t say “the radicals cancel out” in the mechanism. Say they are intermediates that are made in one step and used up in another.
🎯 Product mixture: further substitution happens because chloromethane can also react. Excess methane favours chloromethane as the main product.