
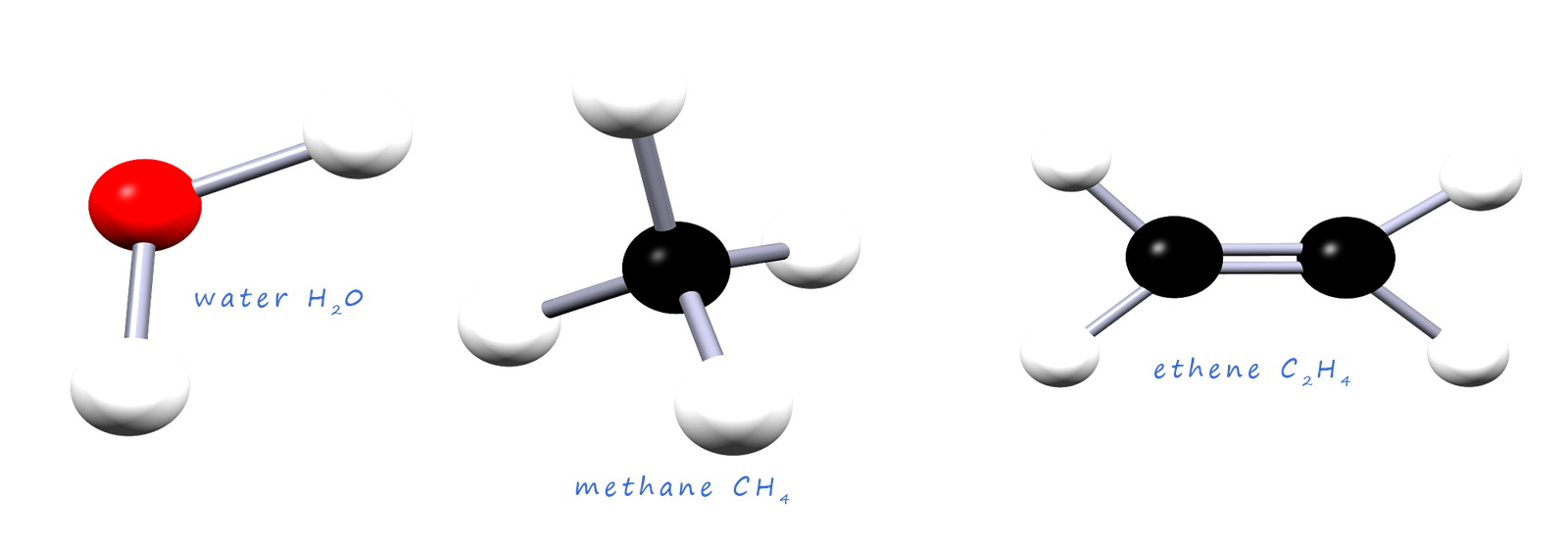
You are no doubt an expert at drawing out the structures for simple molecules like those shown below. In these molecules there are covalent bonds between the individual atoms and as you would expect these covalent bonds involve the sharing of a pair of electrons and the electrons in these covalent bonds are held firmly in place between nuclei of the two atoms involved in the bond.
However there are other molecules such as benzene which cannot be accurately drawn using single
structures like that for methane, water or carbon dioxide shown above. Some of the bonding
electrons in benzene rings
for example are not held in place between the two nuclei
in a covalent bond as in the simple molecules above but they are
delocalised or free to move over multiple atoms.
 These delocalised
electrons are found in a
variety of molecules and ions; not just benzene rings.
Delocalised electrons are generally pi(π)
electrons that are
free to move
over more than two atoms, this leads to a bit
of a problem! How do we draw the structure of benzene or any other molecule
with delocalised electrons
if it is not possible to draw the
position of the bonds holding the molecule together?
These delocalised
electrons are found in a
variety of molecules and ions; not just benzene rings.
Delocalised electrons are generally pi(π)
electrons that are
free to move
over more than two atoms, this leads to a bit
of a problem! How do we draw the structure of benzene or any other molecule
with delocalised electrons
if it is not possible to draw the
position of the bonds holding the molecule together?
In a molecule such as ethene which contain one carbon carbon double covalent bond (C=C), the double covalent bond between the carbon atoms consists of a sigma bond formed by the complete overlap of two atomic orbitals and a pi(π) bond formed by the partial overlap of two p-orbitals on the carbon atoms. The two electrons in each of the sigma and the pi(π) bonds are held firmly between two nuclei in the carbon atoms, this makes it easy to draw out a molecular structure like the one below; we can easily show where the valency electrons are in the molecule by simply looking at the positions of the covalent bonds.
Similarly in other molecules such as oxygen and nitrogen which also contain sigma and pi(π) bonds it is easy to see that the valency electrons are held in fixed positions between the nuclei involved in forming the covalent bonds present in these molecules.
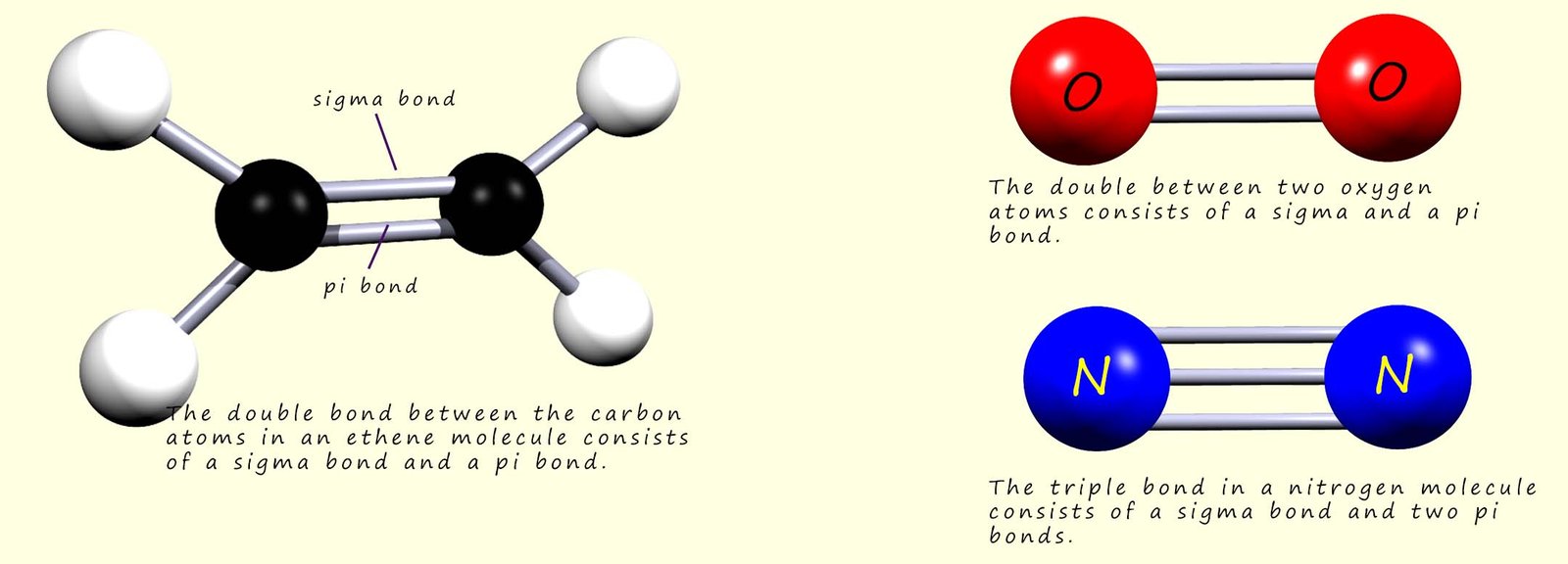
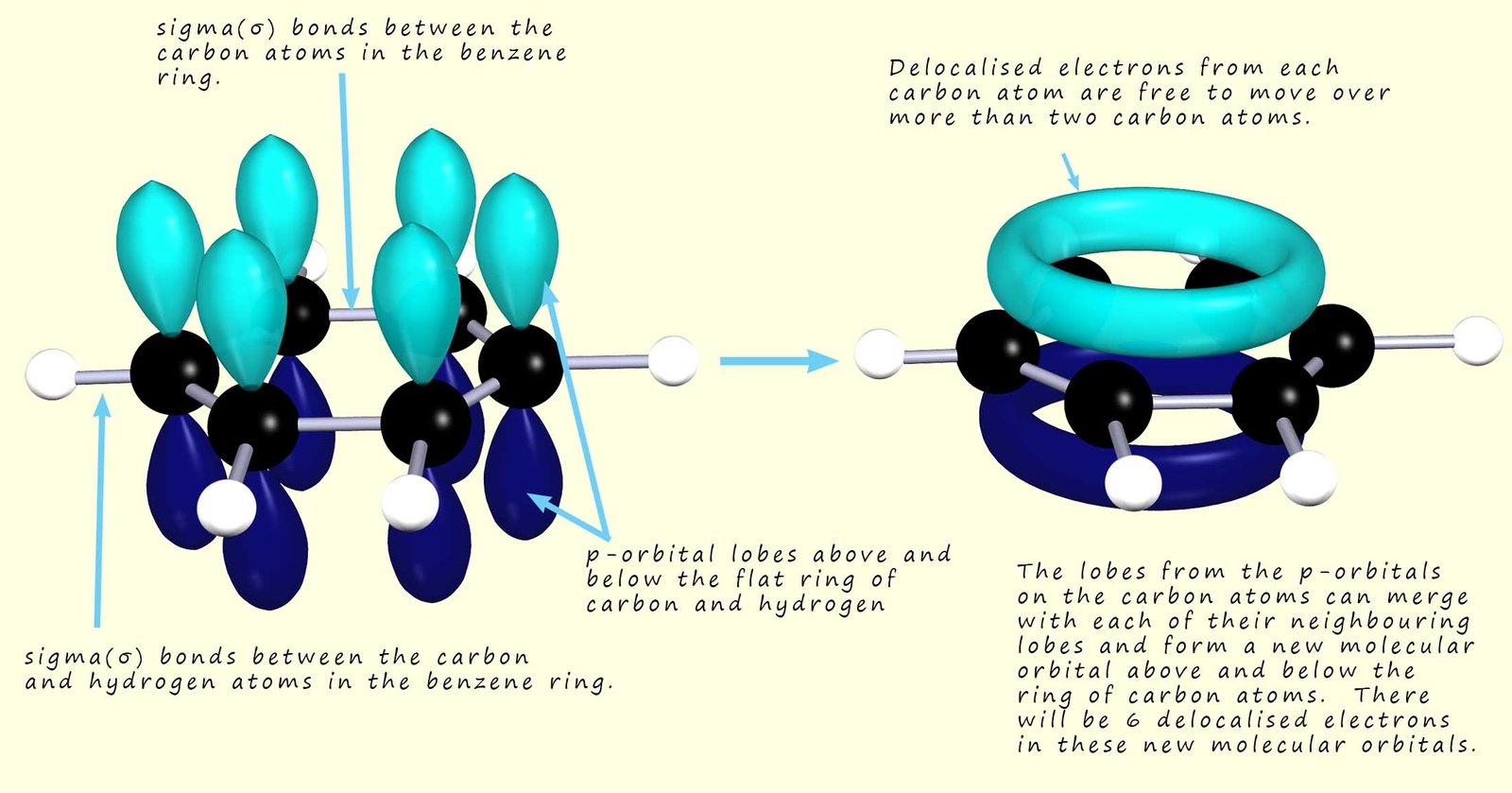
However in a molecule such as benzene the six carbon atoms pi(π) electrons are spread over all six carbon atoms in the benzene ring, that is to say they are delocalised in clouds of electron density above and below the flat planar ring of carbon atoms, this is shown in the image below. This is where the idea of resonance comes into play, resonance theory attempts to describe the delocalisation of electrons within a molecule or ion where the bonding cannot be expressed by one single structure that represents the true electron distribution within the molecule or ion.
To try and draw the structure of a benzene molecule
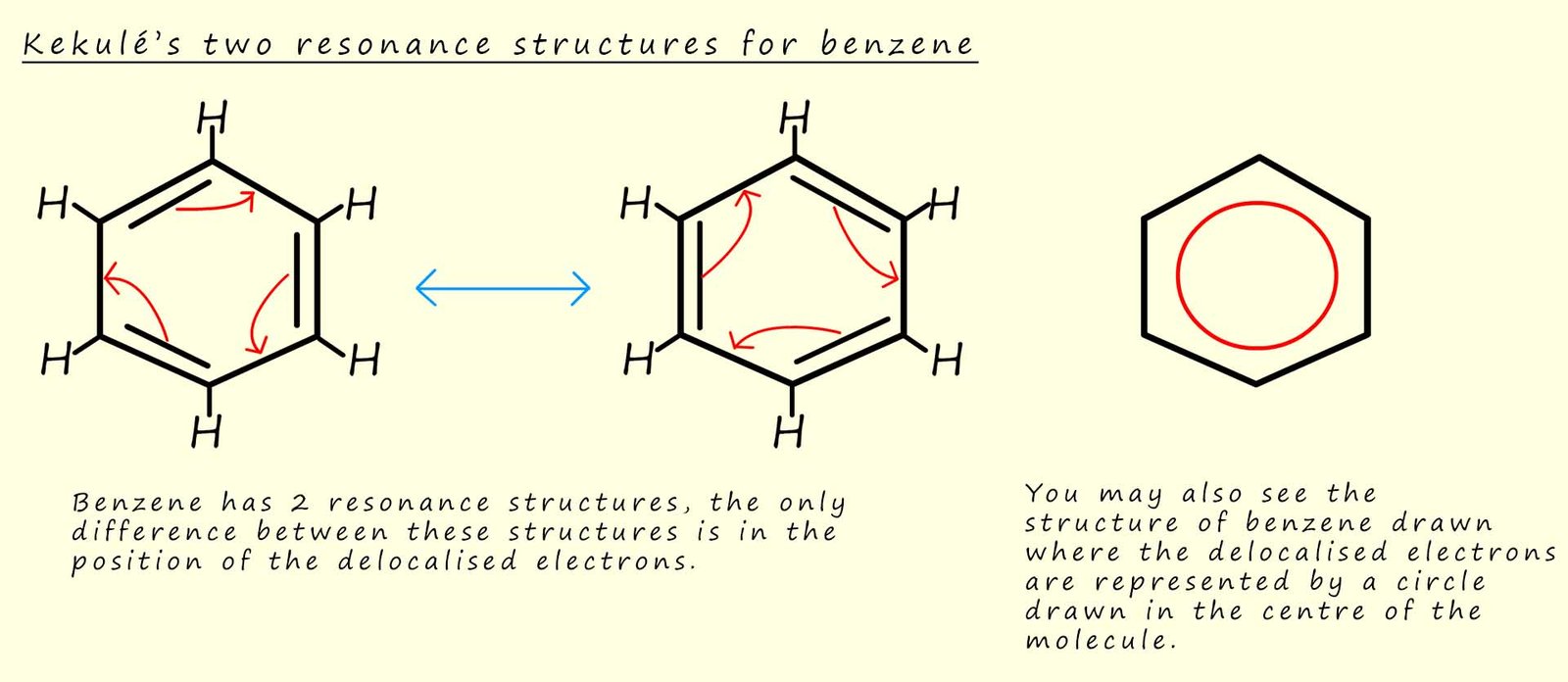
we often draw out two separate structures, as shown in the image below. The two Kekulé structures below for benzene are almost identical to each other apart from the fact that they differ only in the arrangement of their electrons. So which molecule would you draw to represent the true structure of a benzene molecule?
To help you answer this question we can use the idea of resonance. Resonance theory is used to describe the delocalisation of electrons within a molecule where the bonding within the molecule cannot be drawn or represented by one single structure that represents the true electron distribution within the molecule. While the electrons may be delocalised within any one resonance structure, the nuclei of the atoms involved in the bonding remain fixed and do not move. Resonance theory would suggest that the actual structure of benzene is a combination or a blend of the two Kekulé structures shown below. Many textbooks use the term resonance hybrid when discussing resonance theory, where a resonance hybrid is a representation of the actual true structure of a molecule or ion; it represents an average or blend of all possible contributing resonance structures.
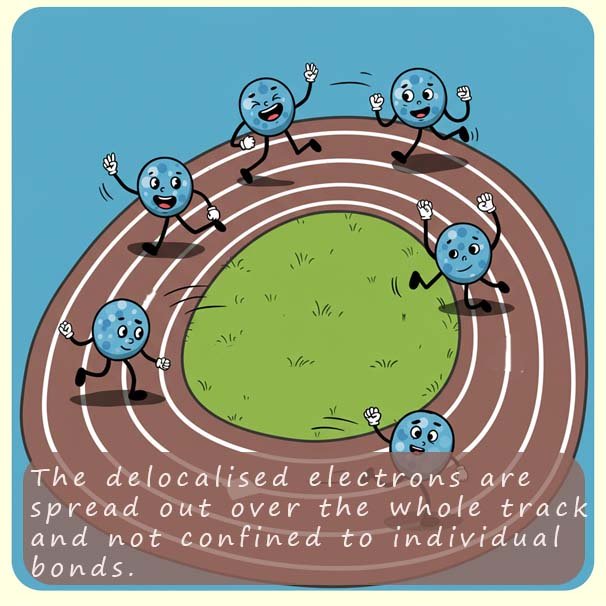 You will also see the structure of a benzene ring drawn as a hexagon with a circle in the centre as shown in the image above. This circle represents the delocalisation of the six pi (π) electrons in the benzene ring; this circle notation provides a way to represent the actual resonance hybrid structure of the benzene ring. A simple analogy which is often used to highlight the significance of this "circle notation" to represent the delocalisation of the six pi electrons in a benzene molecule is- imagine the circle inside the benzene ring as a running track and the runners are represent the delocalised electrons, well in this analogy the runners are not confined to any specific lanes (the bonds) but they can run freely around the entire track in any lane and in any direction. Although you should not imagine delocalised electrons are moving around a molecule, they are simple more spread out or shared across a larger area within the molecule.
You will also see the structure of a benzene ring drawn as a hexagon with a circle in the centre as shown in the image above. This circle represents the delocalisation of the six pi (π) electrons in the benzene ring; this circle notation provides a way to represent the actual resonance hybrid structure of the benzene ring. A simple analogy which is often used to highlight the significance of this "circle notation" to represent the delocalisation of the six pi electrons in a benzene molecule is- imagine the circle inside the benzene ring as a running track and the runners are represent the delocalised electrons, well in this analogy the runners are not confined to any specific lanes (the bonds) but they can run freely around the entire track in any lane and in any direction. Although you should not imagine delocalised electrons are moving around a molecule, they are simple more spread out or shared across a larger area within the molecule.
It is not only in benzene that we encounter the problem of accurately drawing the structure of a particular molecule or ion. If it is possible to describe the structure of a particular molecule or ion in such a way that the only difference between the structures is in the position of the electrons then the actual structure will be a composite or hybrid of the separate resonance structures, that is the actual structure is a resonance hybrid of all the separate resonance structures.
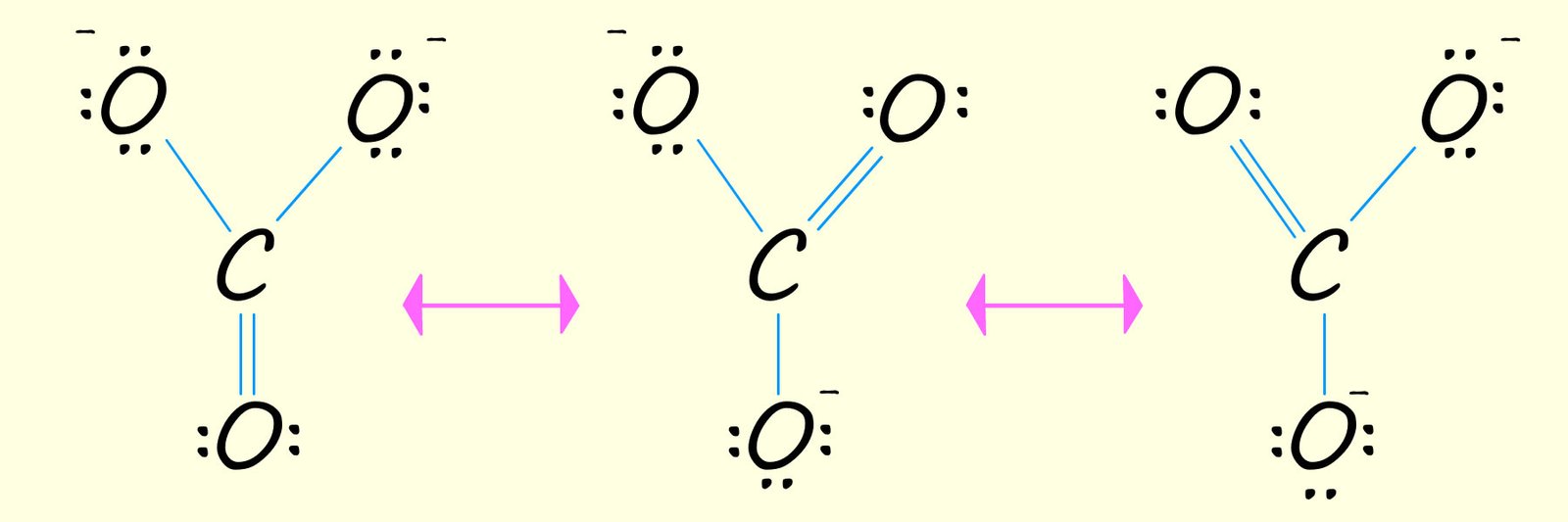
The carbonate ion (CO32-) is another example of a molecule that cannot be drawn out using a single structure. Since carbon atoms make four covalent bonds and oxygen atoms form two covalent bonds we might initially try to draw out the structure of the carbonate ion as a molecule containing two C-O and one C=O, with two of the oxygen forming and ion with a negative charge as shown below. However all the C-O bond lengths in the carbonate ion are the same length, so it cannot consist of single C-O bonds and a C=O bond. The actual structure of the carbonate ion will be a blend or hybrid of the three resonance structure shown in the image below.
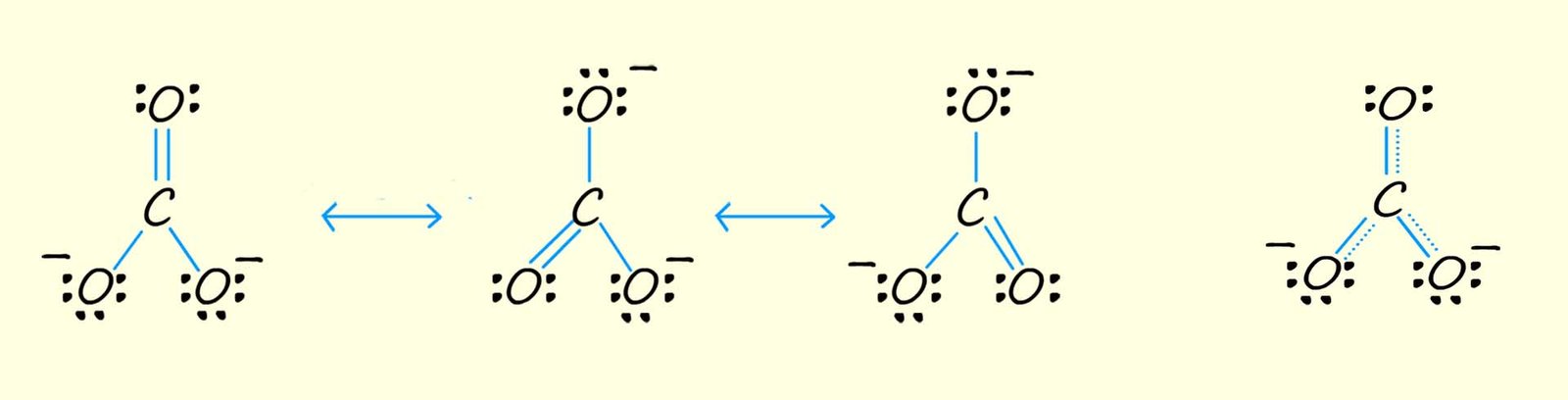
The carbonate ion (CO32-) contains three carbon oxygen bonds which are all the same length, so this means that there cannot be a mixture of single C-O and C=O bonds present within the molecule. The actual structure of the carbonate ion is a mixture or hybrid of all three resonance structures shown above. You may also see the structure of many molecules which undergo resonance drawn with dotted lines, as shown in the fourth drawing of the carbonate ion above, this is to try and represent the delocalised electrons across all the individual resonance structures.
To move from one resonance form to another it is simply a matter of moving an electron pair. In the diagram below the red arrows show the movement of a pair of electrons as one resonance form is changed into another in the forward direction. However you should bear in mind that these electron shifts DO NOT actually take place, remember that the electrons in the ACTUAL carbonate ion are delocalised. The drawing of these resonance structures is simply a limitation of the method used to draw the structures of these molecules.
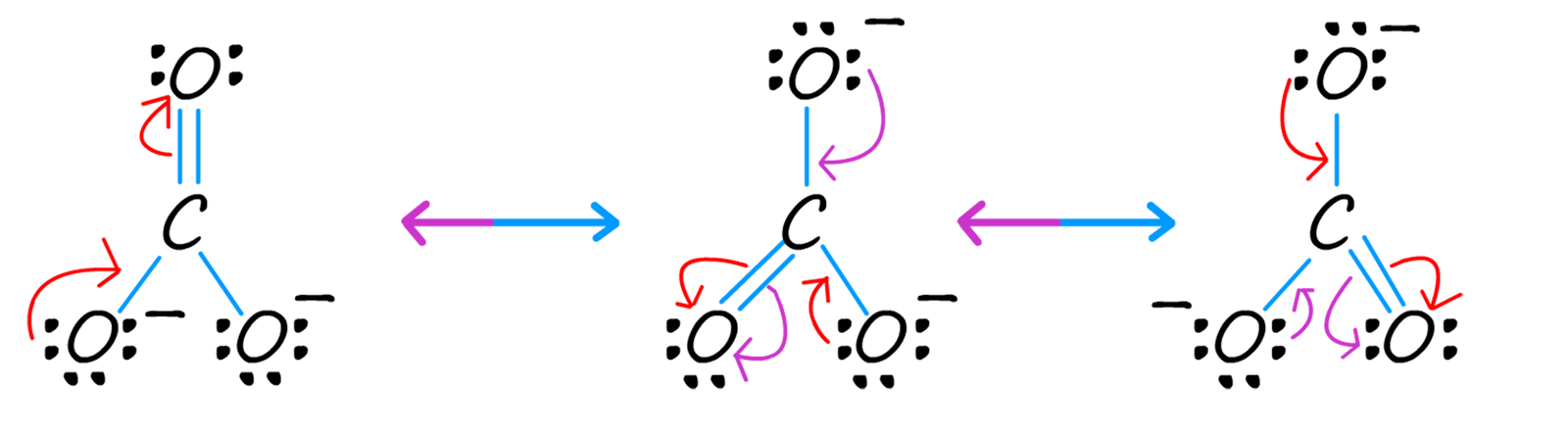
To move from one resonance form of the carbonate ion to another we simply move an electron pair, as shown in the diagram above. The red arrows in the above diagram show the movement of an electron pair in the forward direction while the purple arrows show movement in the reverse direction.
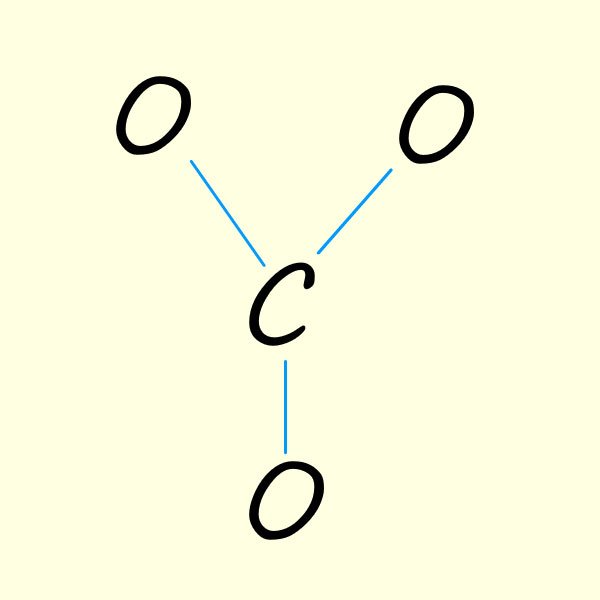
If you are unsure on how to start drawing resonance structures for molecules or ions a good place to start is drawing out Lewis structures. A Lewis structure should show the positions of the valency electrons within the molecule or ion. So for the carbonate ion we have:
So to try and work out the structure of the carbonate ion following the method outlined below:
 |
 |
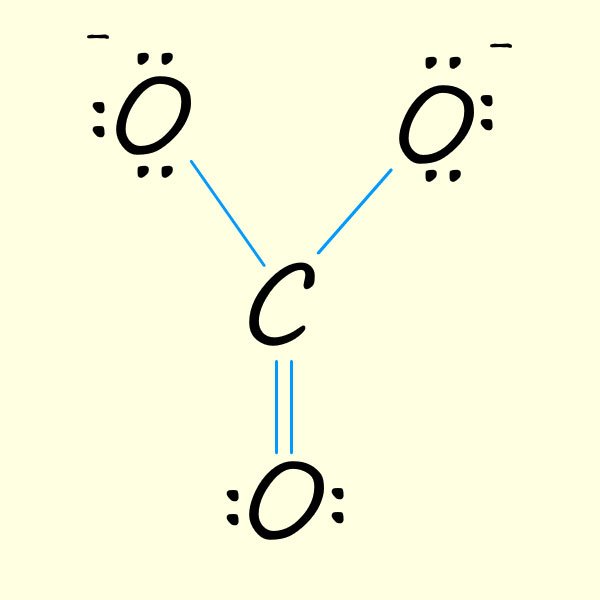 |
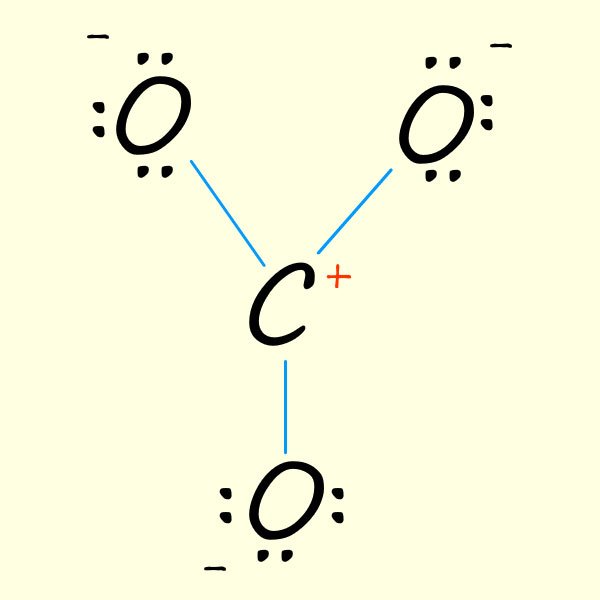
| 1. Start with a basic outline of a possible structure for the carbonate ion. Here we have used up 6 valency electrons in the three covalent bonds formed between the oxygen atoms and the carbon atom. One immediate problem here the the central carbon atom is only making 3 bonds when carbon atom s have a valency of 4 and so should form 4 covalent bonds. | 2. Now place the remaining 18 valency electrons in the molecule. Here I just placed them on each oxygen. This gives each oxygen 8 electrons in the valency shell, that is it has gained an electron and so each will have a - charge. The carbon atom, a group 4 element only has 3 valency electrons in the above and so will have a + charge. | 3. To ensure carbon has an octet of electrons simply move electrons from one of the oxygen atoms to form one C=O bond. However you can obviously move the electrons from any of the 3 oxygen atoms to give each of the resonance structure shown below: |
From point 3 in the table above it is simply a matter of shifting the electrons present on each of the negatively charged oxide ions (O2-) to obtain each of the possible resonance structures for the carbonate ion (CO32-) as shown below, recall that the double headed arrow in the image below shows resonance, that is the movement of delocalised electrons while the nuclei of the atoms remain in fixed positions:
As another example of resonance consider aromatic amines. Aliphatic amines and ammonia are good bases due to the presence of the lone pair of electrons on the nitrogen atom which is able to form dative covalent bonds with hydrogen ion (H+) in acids. As a simple example consider ammonia (NH3) which can use it lone pair of electrons to form a dative covalent bond to a hydrogen ion (H+), as shown in the image below:

However aromatic amines such as phenylamine or aniline (C6H5NH2) are poor bases. In an aromatic amine the nitrogen atom is bonded directly to an aromatic ring, this means that the lone pair of electrons on the nitrogen atom can be delocalised through the aromatic ring and so they are not readily available to form dative covalent bonds in the same way aliphatic amines can. This is outlined in the diagram below:
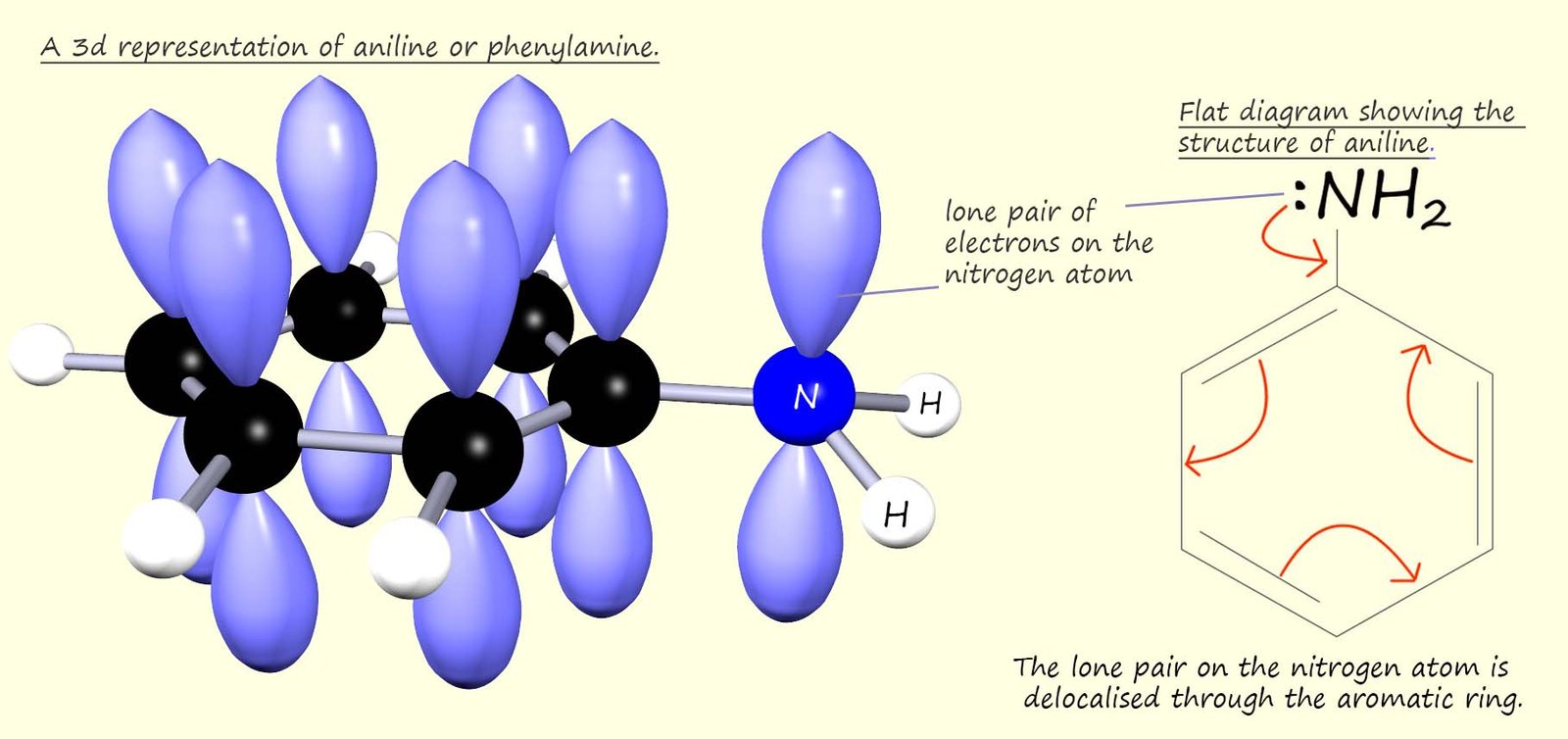
In aniline the NH2 group is attached directly to an aromatic ring. The aromatic ring contains 6 delocalised electrons, one from each carbon atom present in the aromatic ring. The p-orbitals on the carbon atoms in the ring can merge with the p-orbital on the nitrogen atom, this means that the lone pair of electrons on the nitrogen atom will be delocalised through the aromatic ring. This means that the lone pair of electrons on the nitrogen atom is delocalised through the aromatic ring system and so they will not be available to form dative covalent bonds, that is aromatic amines are poor bases.
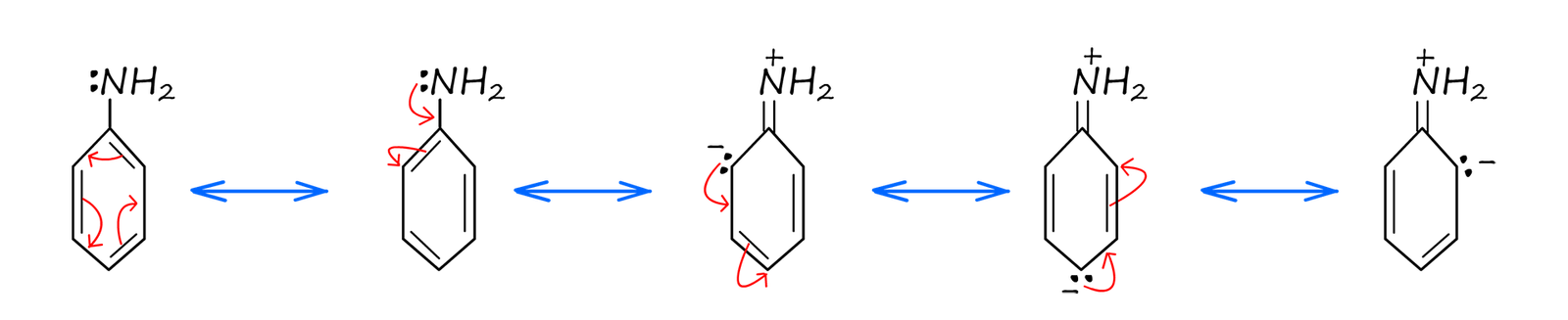
The delocalisation of the pi(π) electrons will lead to the same issues we had above when trying to draw the structure of a benzene molecule. Delocalisation implies that the electrons are not in fixed positions so trying to draw molecular structures and bonds will not be possible. Instead we have to try and show the structure of aniline by drawing a series of resonance structures, shown below. Recall that the actual structure of aniline will be a mix or composite of all the individual resonance structures.
Match the term below with the correct definition by simply clicking on the term and then its correct definition. Correct responses will turn the definition green!
Click or tap the flashcards below.