
Addition of bromine water to a substance is often used to test for unsaturation in a molecule, for example the addition of bromine water to an unsaturated alkene in a boiling tube will instantly result in the decolourisation of the reddish-brown bromine water. In the image below the left-hand test tube contains cyclohexane (C6H12); a saturated hydrocarbon while the right-hand test tube contains cyclohexene (C6H10); an unsaturated hydrocarbon.
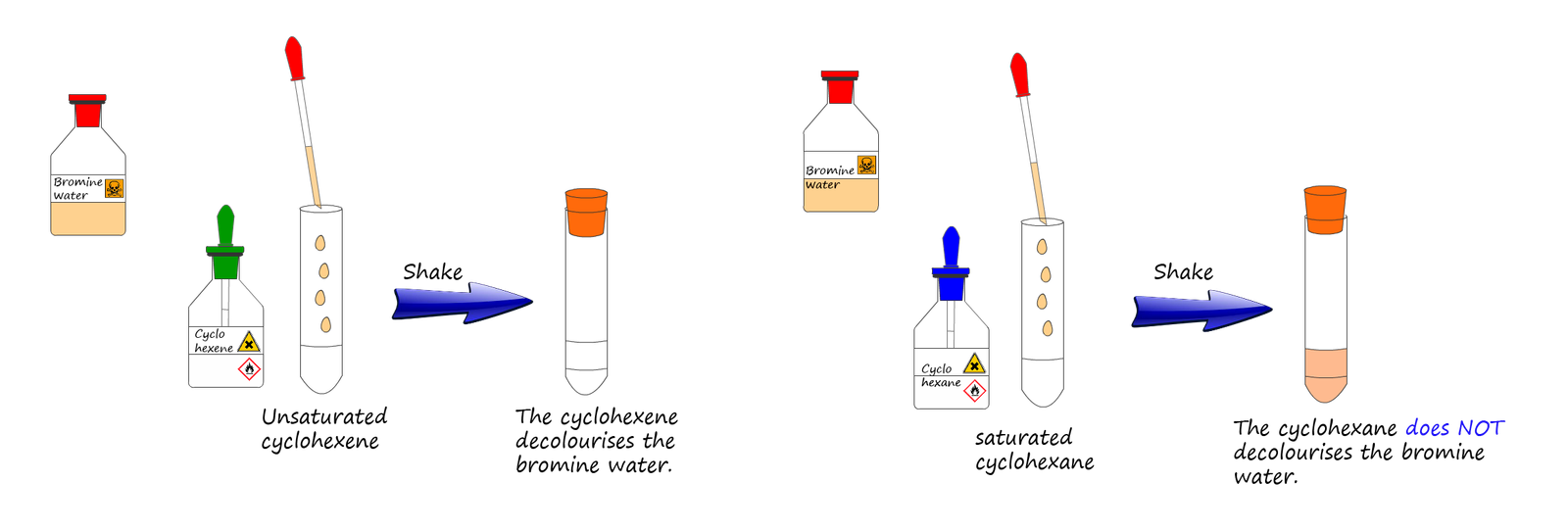
If a small amount of bromine water is added to each test tube and then both test tubes are given a quick shake to mix the contents together, then the bromine water is instantly decolourised in the test tube containing the unsaturated cyclohexene while the bromine water stays orange in the test tube containing the saturated cyclohexane.
It is worth mentioning that the orange colour of the bromine will switch layers in the test tube containing the saturated cyclohexane. Halogens such as bromine are more soluble in organic solvents than in water, so when the contents of the test tube are shaken up, although no chemical reaction will occur immediately the majority of the bromine will move into the top less dense organic layer of cyclohexane while the aqueous layer which initially contained the bromine dissolved in it will turn clearer as the bromine dissolves in the organic cyclohexane layer, this is shown in the left hand test tube in the image above.
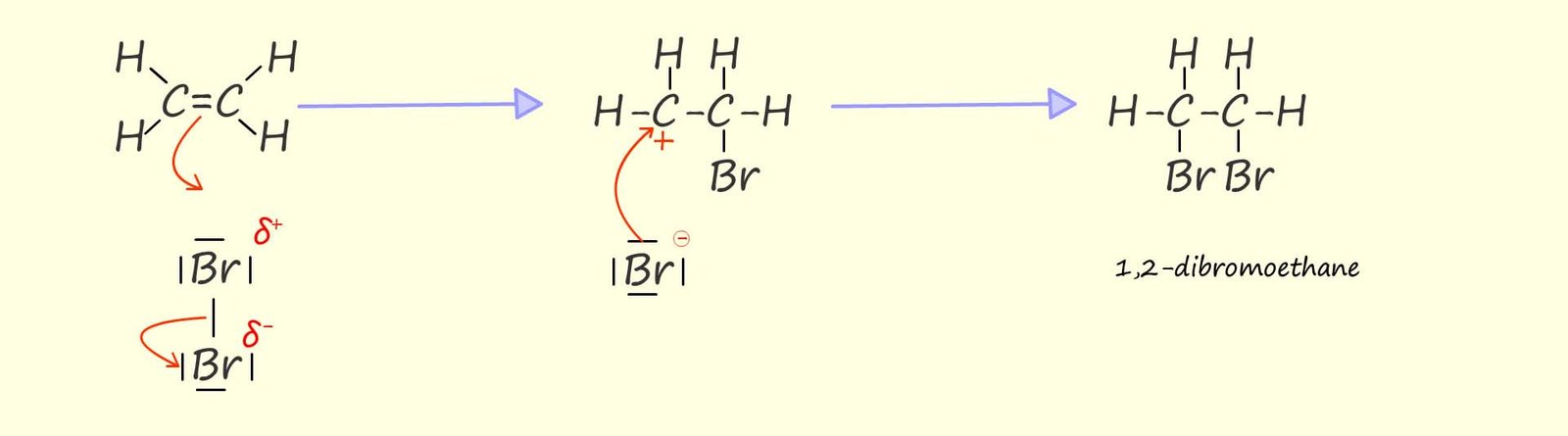
As you may recall alkenes under electrophilic addition reactions. The mechanism below shows
how bromine adds to the carbon carbon double bond in the unsaturated alkene ethene. You may recall that:
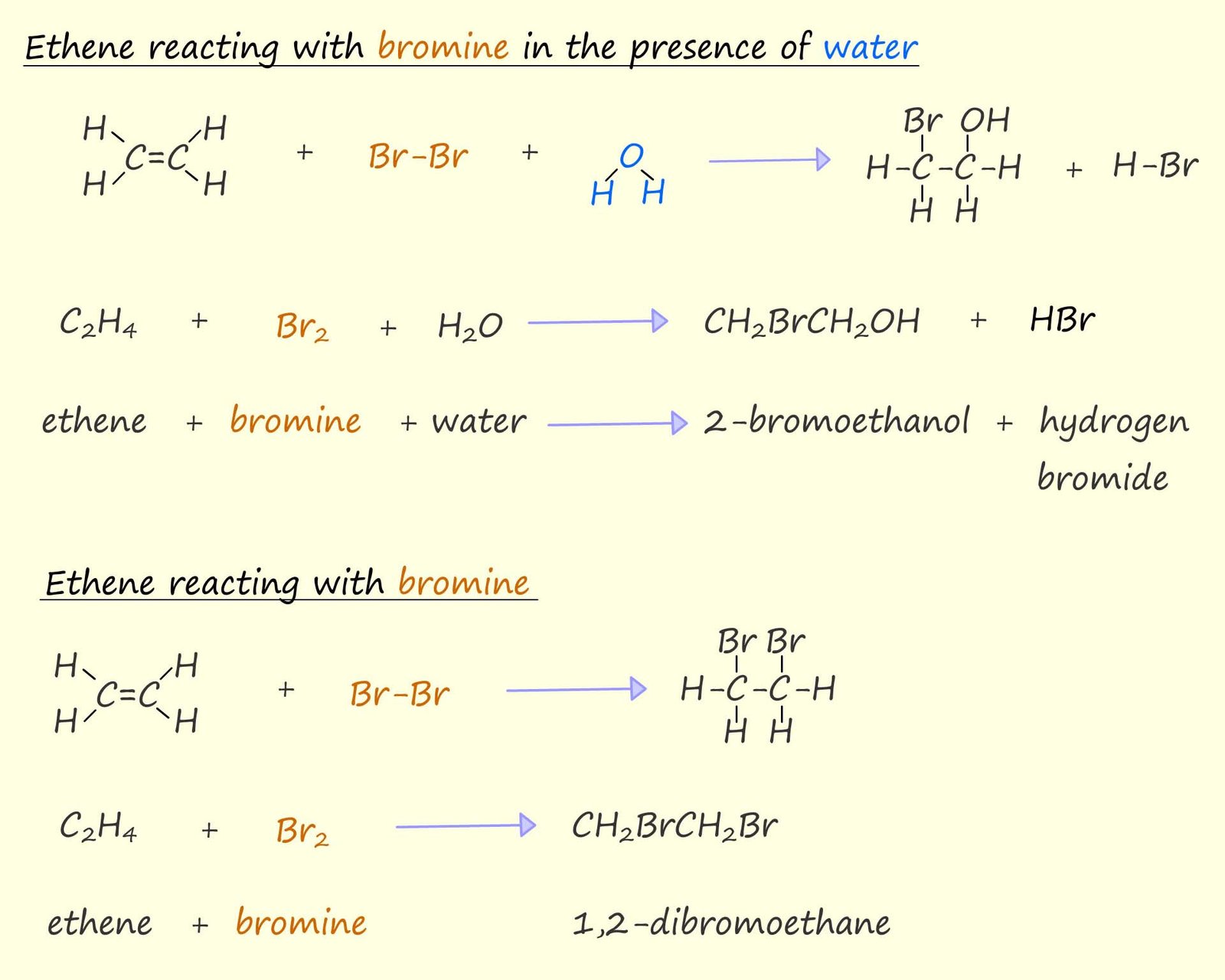
However if the ethene is swapped for benzene or any another aromatic compound one difference in the reaction with bromine or bromine water is immediately obvious; aromatic rings will not react with bromine or other halogens, they are much less reactive than alkenes towards electrophiles. I am sure you are aware that while unsaturated molecules such as alkenes readily undergo electrophilic addition reactions aromatic molecules such as benzene will not undergo electrophilic addition reactions but instead undergo electrophilic substitution reactions.
In the image below bromine has been dissolved in an organic solvent such as chloroform or carbon tetrachloride, it is then added to a test tube containing benzene and another test tube containing the unsaturated hydrocarbon cyclohexene (C6H10). Both test tubes are stoppered and shaken. The results are shown in the image. Cyclohexene rapidly decolourises the orange bromine solution but in the test tube containing benzene the orange colour due to presence of bromine persists and does not fade.
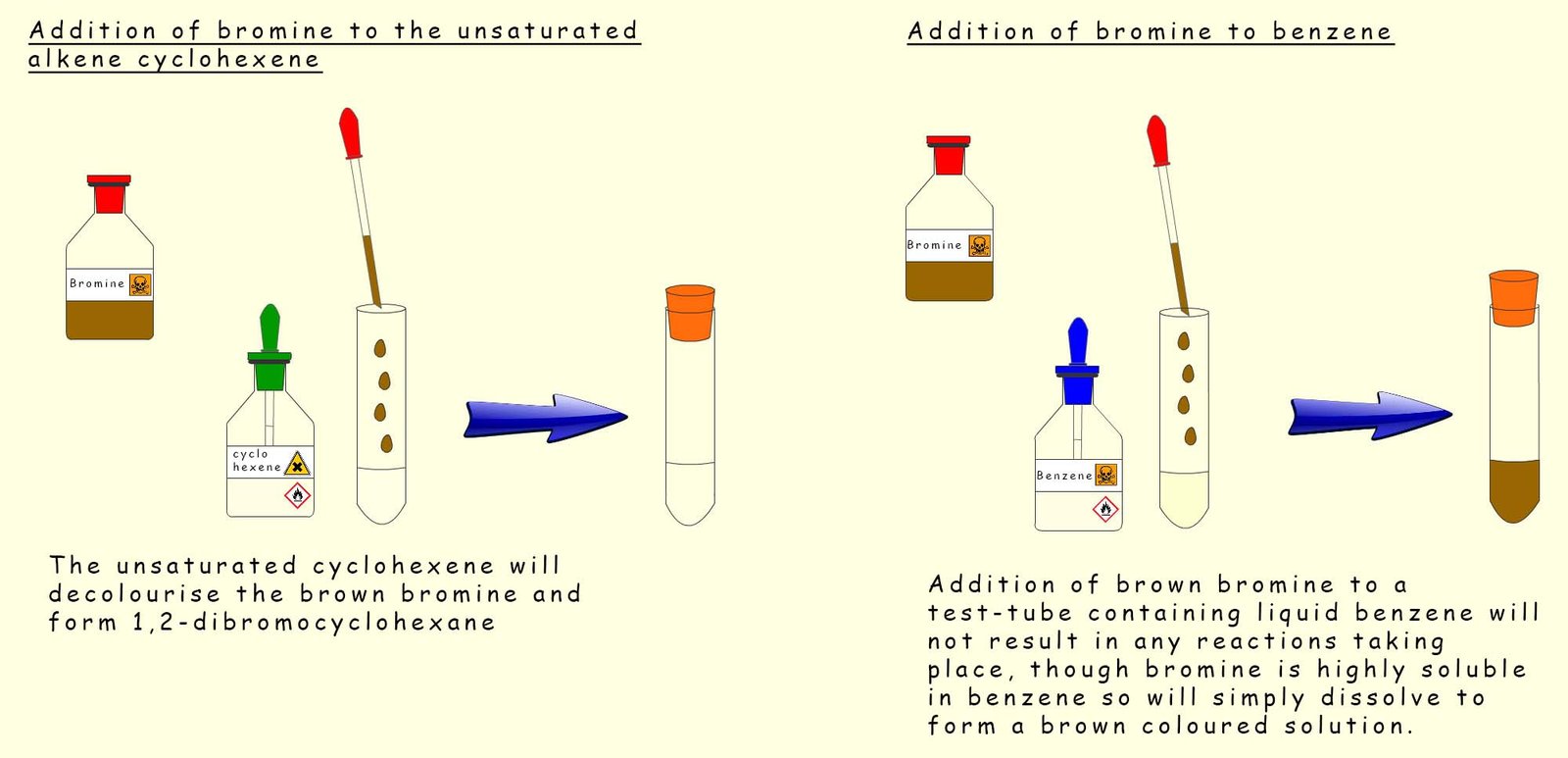
As mentioned above if a solution of bromine in an organic solvent is added to a test tube containing benzene then no reaction occurs, the orange colour due to the presence of bromine persists. However if some iron filings or iron (III) bromide are added to the test tube then the orange colour due to the presence of bromine decolourises and white misty acidic fumes of hydrogen bromide are observed above the test tube. If a piece of moist pH paper or blue litmus paper is held above the test tube it turns red due to the acidic hydrogen bromide gas which is released. Equations for these reactions are shown below:
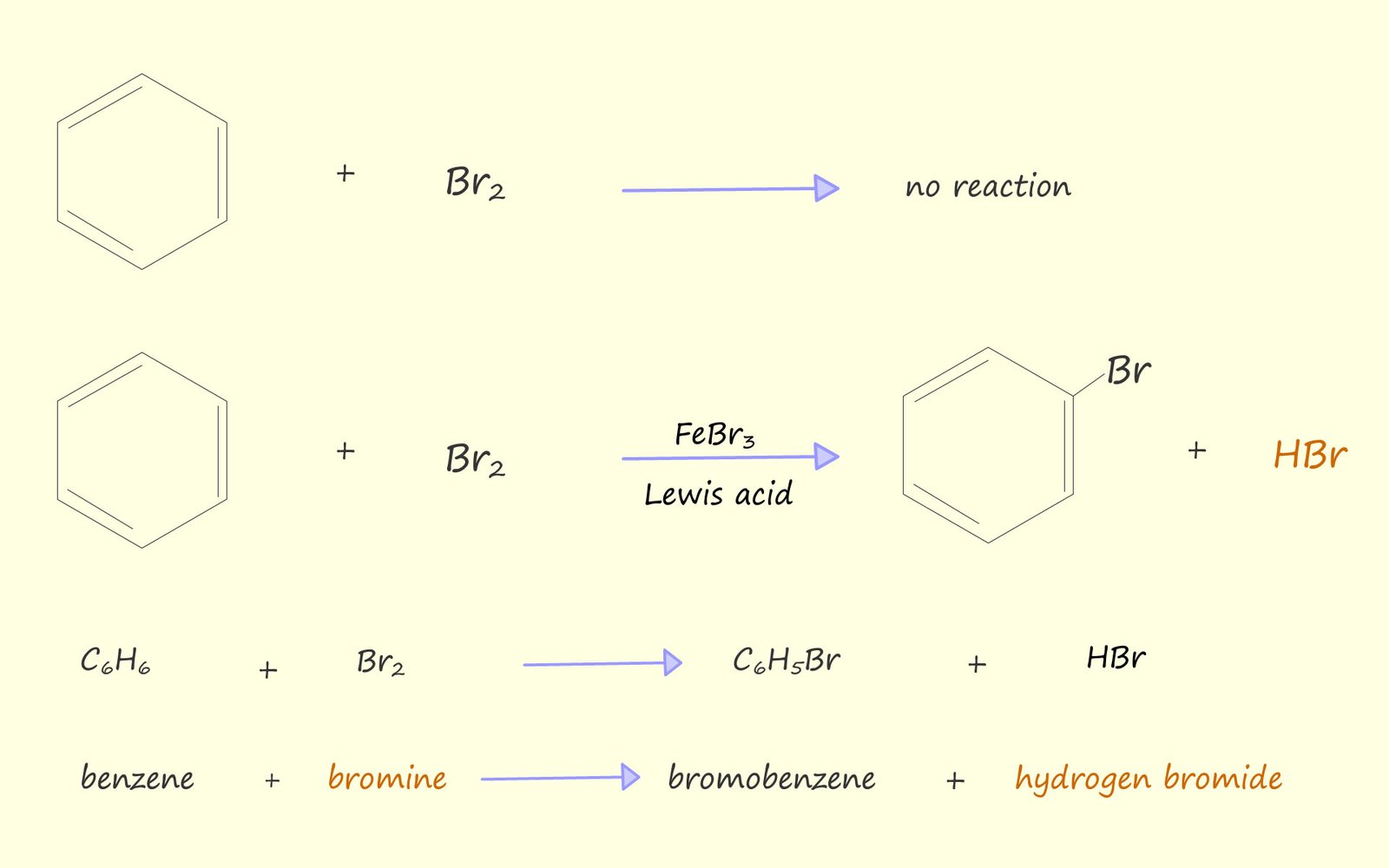
From the equations above I hope you can see that the bromine swaps or substitutes for one of the hydrogen atoms on the benzene ring. The reaction as you might expect from an aromatic ring is electrophilic substitution.
 Why then do unsaturated alkenes undergo electrophilic addition reactions but aromatic rings such as benzene undergo electrophilic substitution reactions instead?
Well the pi(π) electrons in an alkene molecule are localised between two carbon atoms, whereas in an aromatic molecule such as benzene
the pi(π) electrons are delocalised over all of the carbon atoms, this delocalisation makes benzene and other aromatic molecules very stable and means basically that in order for an electrophile to react with benzene or another aromatic molecule it needs to be an extremely "good electrophile".
Why then do unsaturated alkenes undergo electrophilic addition reactions but aromatic rings such as benzene undergo electrophilic substitution reactions instead?
Well the pi(π) electrons in an alkene molecule are localised between two carbon atoms, whereas in an aromatic molecule such as benzene
the pi(π) electrons are delocalised over all of the carbon atoms, this delocalisation makes benzene and other aromatic molecules very stable and means basically that in order for an electrophile to react with benzene or another aromatic molecule it needs to be an extremely "good electrophile".
Non-polar molecules such as bromine will NOT react with aromatic rings, simply because they are "not good" enough electrophiles. For this reason a catalyst or a halogen carrier as they are often referred to are needed to increase the ability of a species to act as an electrophile. The delocalisation of the π-electrons as mentioned results in an
increase in the stability of the benzene ring and this will raise the activation energy for any reaction which will destroy this delocalisation energy.
We have seen from the equations above that benzene will react with bromine or indeed chlorine and iodine in the presence of catalysts such as iron or even aluminium. These metals react with the halogens to form metal (III) halides such as aluminium chloride (AlCl3) or iron (III) bromide. This is shown in the equations below:
The catalysts or halogen carriers are they are often called work by producing much more powerful electrophiles.
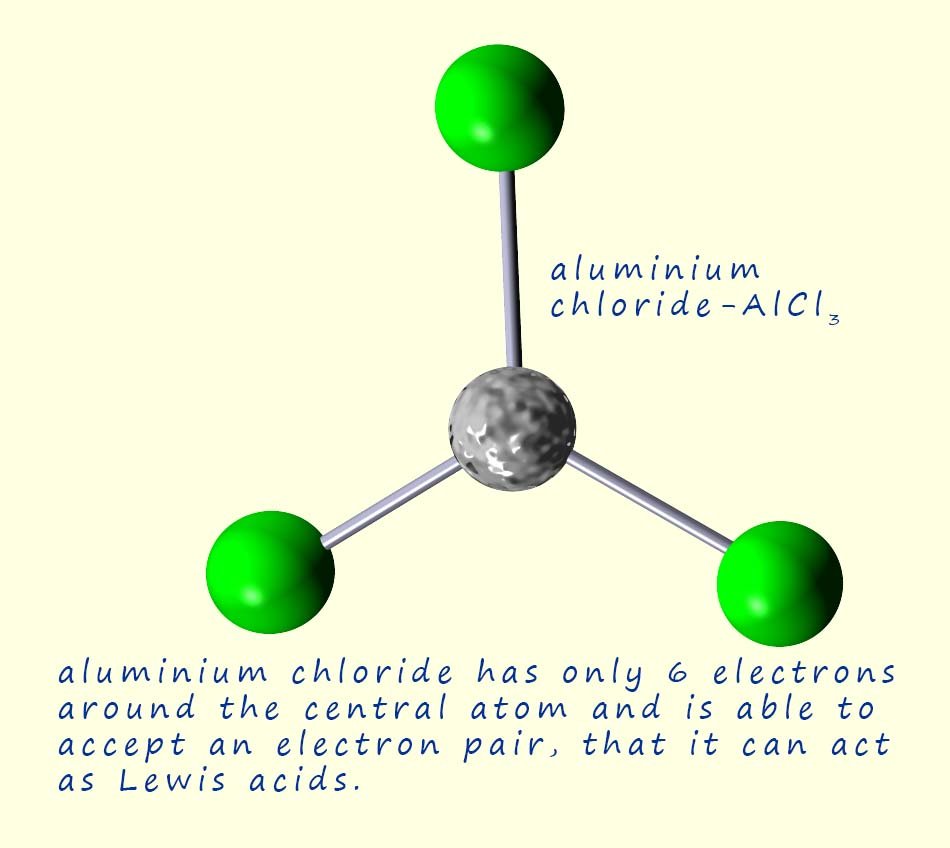 Lewis acids are substances which are able to accept a pair of electrons from another species; that is a Lewis base. In order to accept a pair of electrons the Lewis acid must have
a space to put them! That is it must have empty orbitals available. Iron (III) ions (Fe3+) for example have empty d-orbitals which can accept an electron pair while aluminium chloride (AlCl3) has only 6 electrons in its valency shell so like iron(II) ions it has empty orbitals which can accept an electron pair.
Lewis acids are substances which are able to accept a pair of electrons from another species; that is a Lewis base. In order to accept a pair of electrons the Lewis acid must have
a space to put them! That is it must have empty orbitals available. Iron (III) ions (Fe3+) for example have empty d-orbitals which can accept an electron pair while aluminium chloride (AlCl3) has only 6 electrons in its valency shell so like iron(II) ions it has empty orbitals which can accept an electron pair.
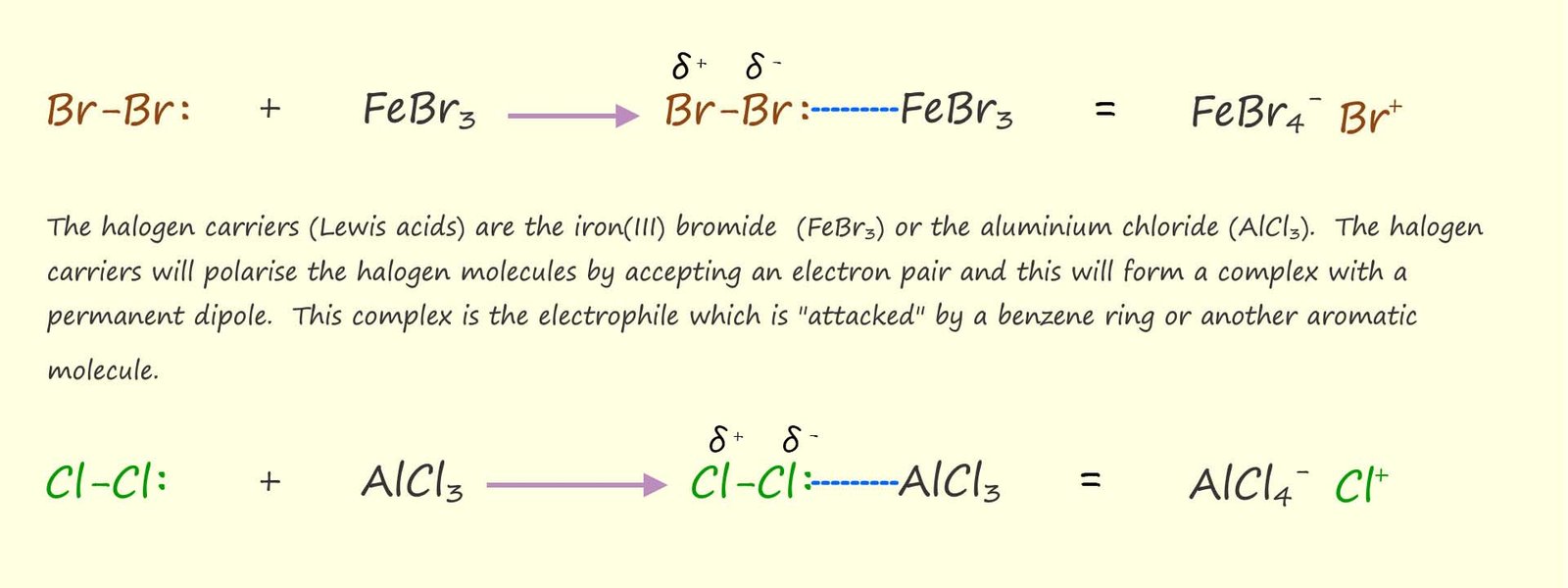
Iron (III) bromide(s) (FeBr3) is the Lewis acid catalyst or halogen carrier usually used to brominate aromatic rings while aluminium chloride (AlCl3) or iron (III) chloride (FeCl3) is usually used to chlorinate aromatic rings. The Lewis acid catalysts works by basically polarising the bromine molecule (Br2) or the chlorine molecule (Cl2) to form a much better electrophile. The image below shows how the Lewis acid or halogen carrier is able to polarise the halogen molecule.
It is the complex formed when the halogen makes a dative covalent bond to the Lewis acid or halogen carrier that acts as the electrophile. The formation of a dative covalent bond between the Lewis acid and the halogen molecule will induce a permanent dipole in the halogen molecule making it a much better electrophile.
Quickly check your understanding of a few of the key terms mentioned above by answering these two short questions:
Aromaticity is a special stability found in certain cyclic compounds where:
Lewis acids or halogen carriers are substances which are able to accept a pair of electrons from another species; that is a Lewis base. In order to accept a pair of electrons the Lewis acid must have a space to put them, that is it must have empty orbitals available.
The halogen carrier will form a dative covalent bond with the halogen molecule which will induce a permanent dipole in the halogen molecule; that is the halogen molecule will have partially charges ends; that is δ+ and δi ends which will make it a much better electrophile.
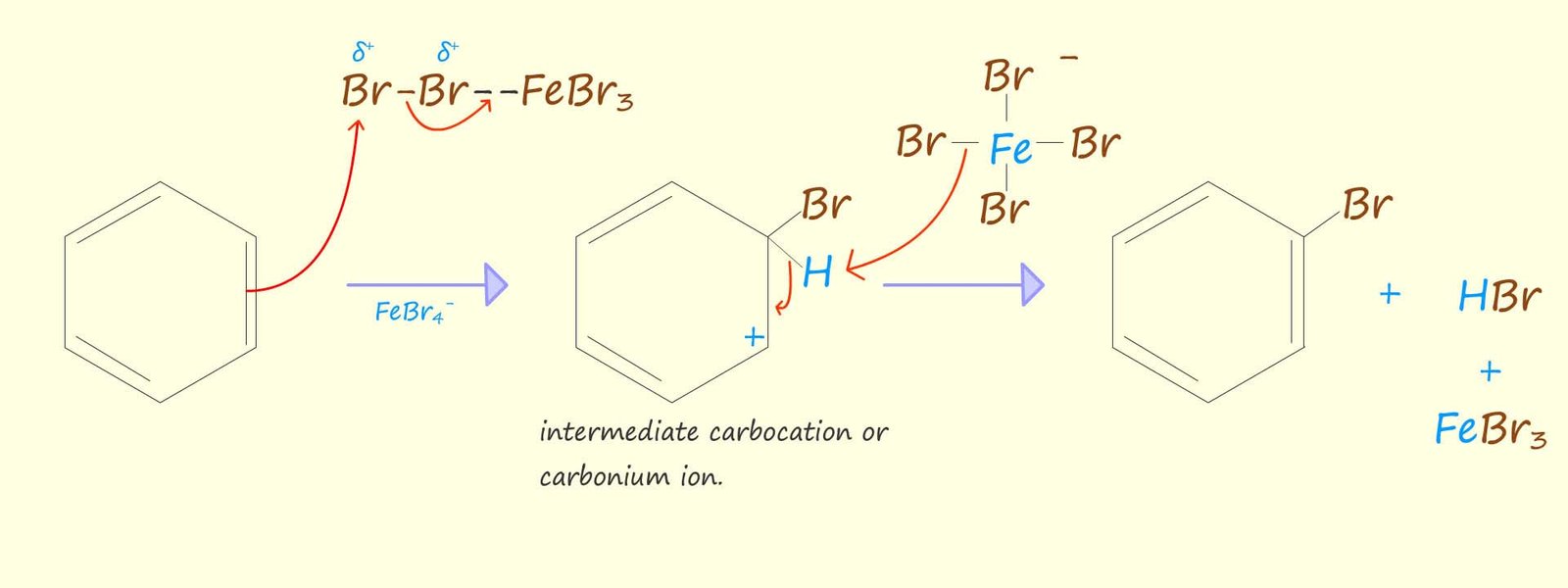
An aromatic molecule such as benzene is able to use its delocalised pi electrons to attack the complex containing the polarised halogen molecule. The mechanism for the bromination of benzene is shown below. It is really looks no different to the other electrophilic substitution reactions that we have seen for other aromatic compounds. The reaction can be broken down into a number of key steps:
Or if you prefer to usethe circle notation to represent the delocalised electrons in benzene we have:
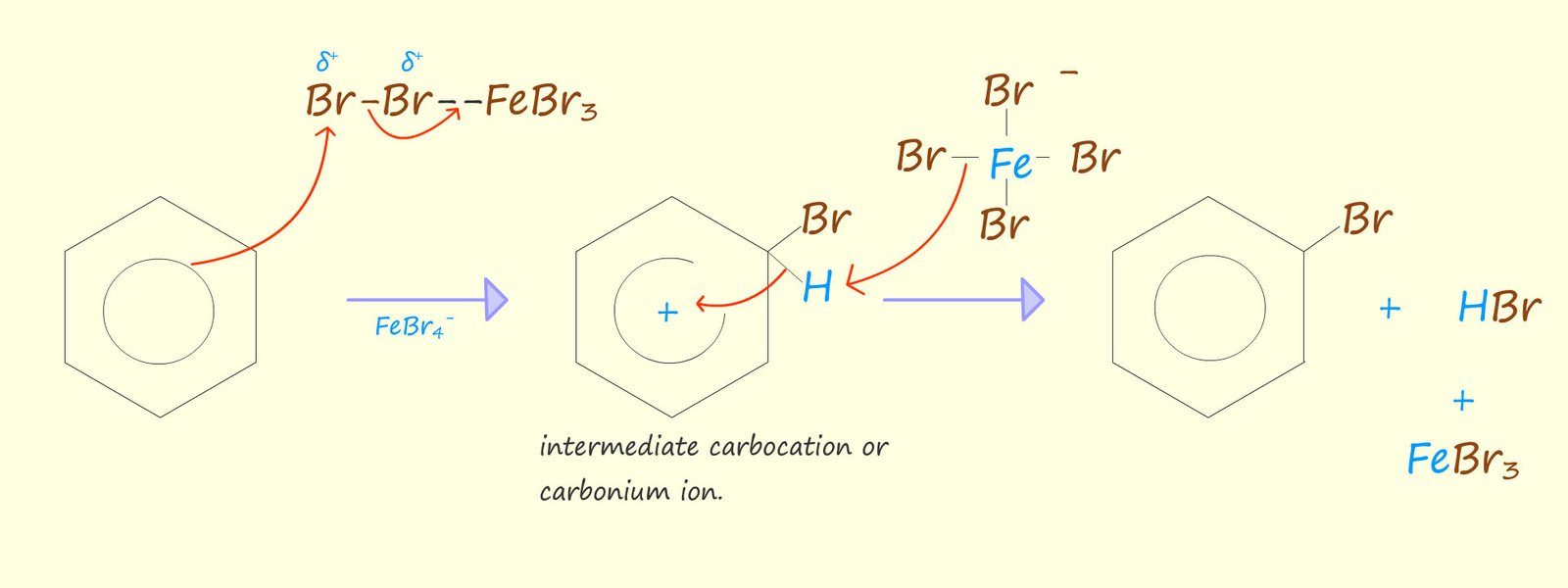
The final step involves the attack of the FeBr4- ion, which helps to remove the hydrogen ion (H+) from the intermediate carbocation. The FeBr4- will react with the hydrogen ion (H+) to regenerate the Lewis acid catalyst- FeBr3 and also form the acidic gas hydrogen bromide (HBr). An equation for this reaction is shown below:
Test your understanding of the main ideas covered above by filling in the blanks to complete the paragraphs below. The words to complete the blanks are shown in the yellow box and the drop down menus. Simply select the correct word or words from the drop-down menus to correctly complete the two short pargraghs.
