

The Friedel-Crafts alkylation reaction is used to add alkyl groups such as methyl (-CH3) and ethyl groups
(-C2H5) on to an aromatic ring. During a Friedel-Crafts reaction the alkyl group replaces or substitutes for one of the hydrogen atoms on the aromatic ring. The type of mechanism as you might expect
from an aromatic ring is
electrophilic substitution. The Friedel-Crafts alkylation reaction is just another example of an electrophilic substitution reaction and can be thought of as occurring in three separate steps:
The equation below outlines an equation that shows a Friedel-Craft reaction using benzene as an example, here the benzene ring reacts with an electrophile (R+) which simply substitutes or replaces one of the hydrogen atoms present on the benzene ring. The electrophile in a Friedel-Crafts reaction is produced by the reaction of a halogenalkane (an alkyl halide) with a Lewis acid catalyst. Aluminium chloride (AlCl3) is a common Lewis acid catalyst used in these reactions.
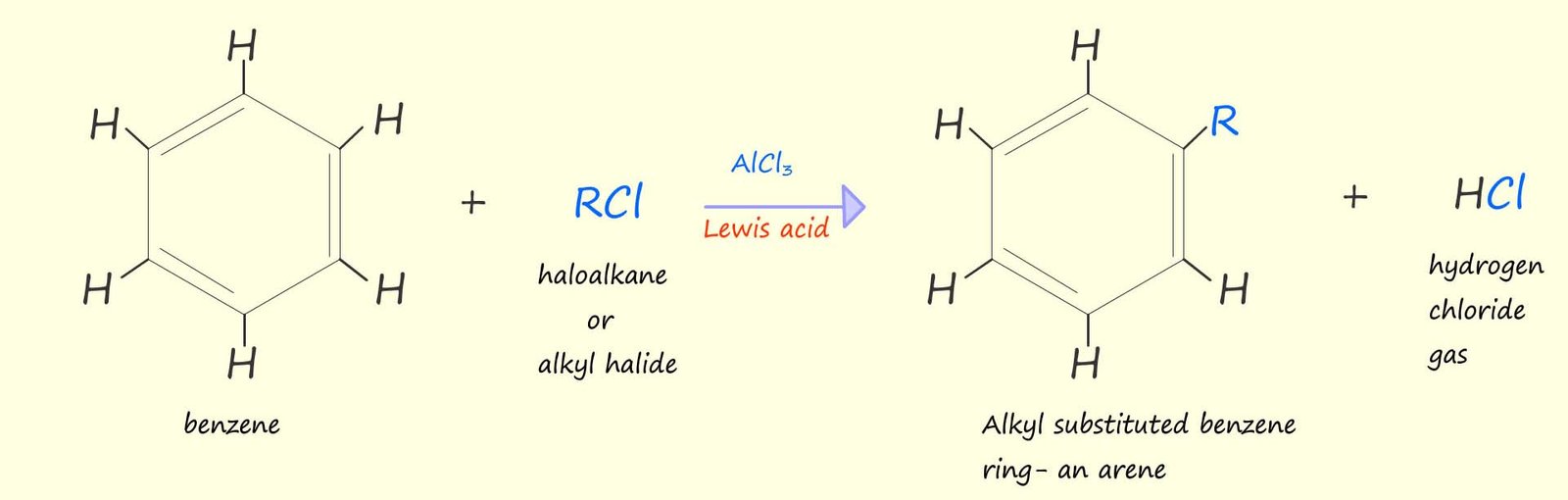
Or if you prefer to use the circle notation to show the benzene ring rather than the Kekulé structure then we have:
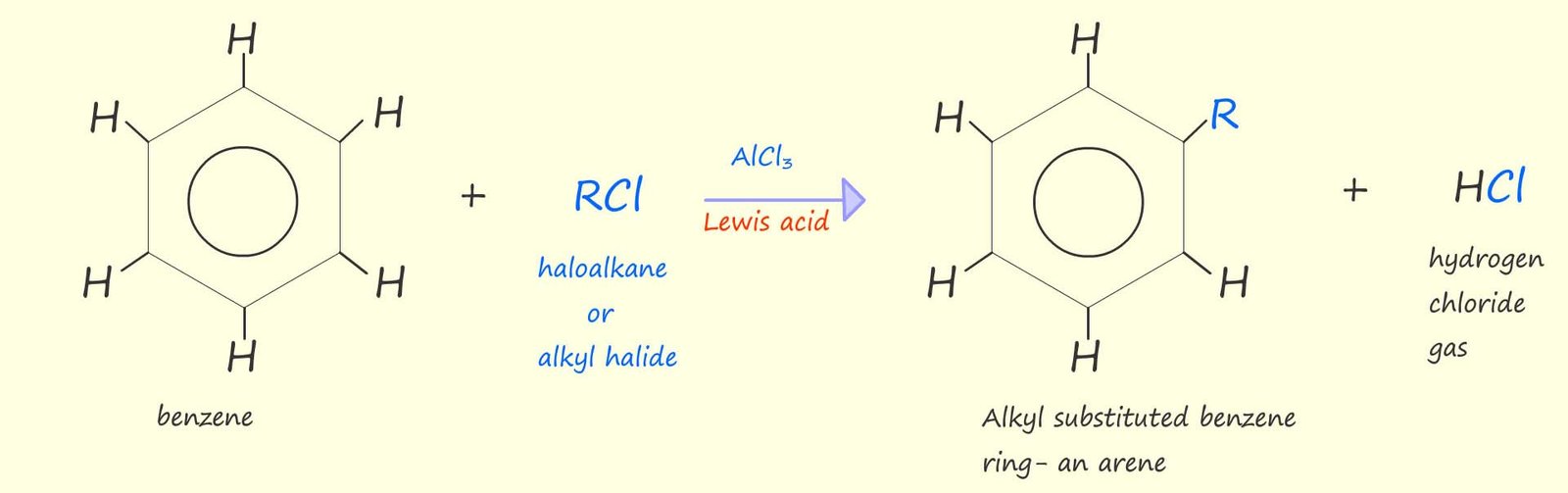

As in any electrophilic substitution reaction the delocalised pi(π) electrons in the aromatic ring act as a nucleophile and attack a carbocation. The positively charged carbocation (the electrophile) in these reactions is generated by the reaction of a halogenalkane (alkyl halide) with a Lewis acid catalyst such as aluminium chloride (AlCl3) or iron (III) chloride (FeCl3) at around 800C.
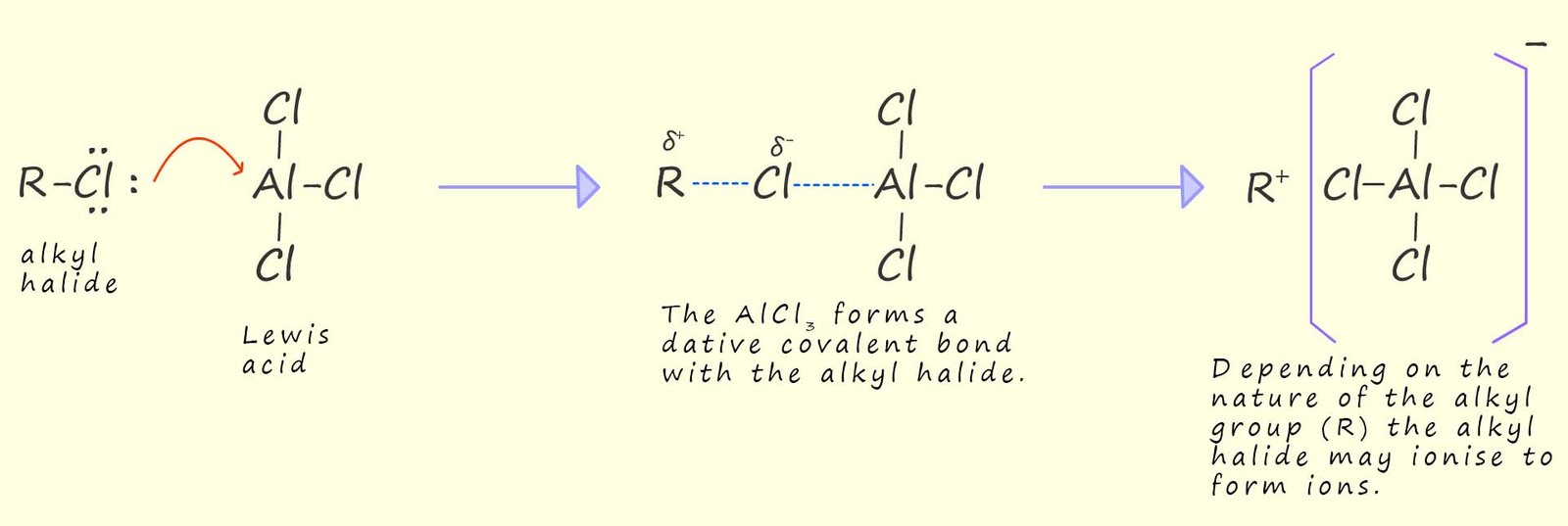
In a Friedel-Crafts alkylation reaction the electrophile is a carbocation which is often given the symbol (R+); it is formed by the reaction of a halogenalkane molecule with a Lewis acid (an electron pair acceptor) such as aluminium chloride (AlCl3) or iron (III) bromide (FeBr3), both of these molecules are electron deficient and have empty orbitals that are capable of accepting a lone pair of electrons.
The halogenalkane molecule (R-X) will supply the carbocation (R+) that will add to the aromatic ring. X is typically a halogen atom such as Cl, Br or I. The steps which take place in forming the carbocation electrophile can be summarised as:
Match up the terms with their correct definitions. Simply click the term and then its correct definition, correct responses will turn green.
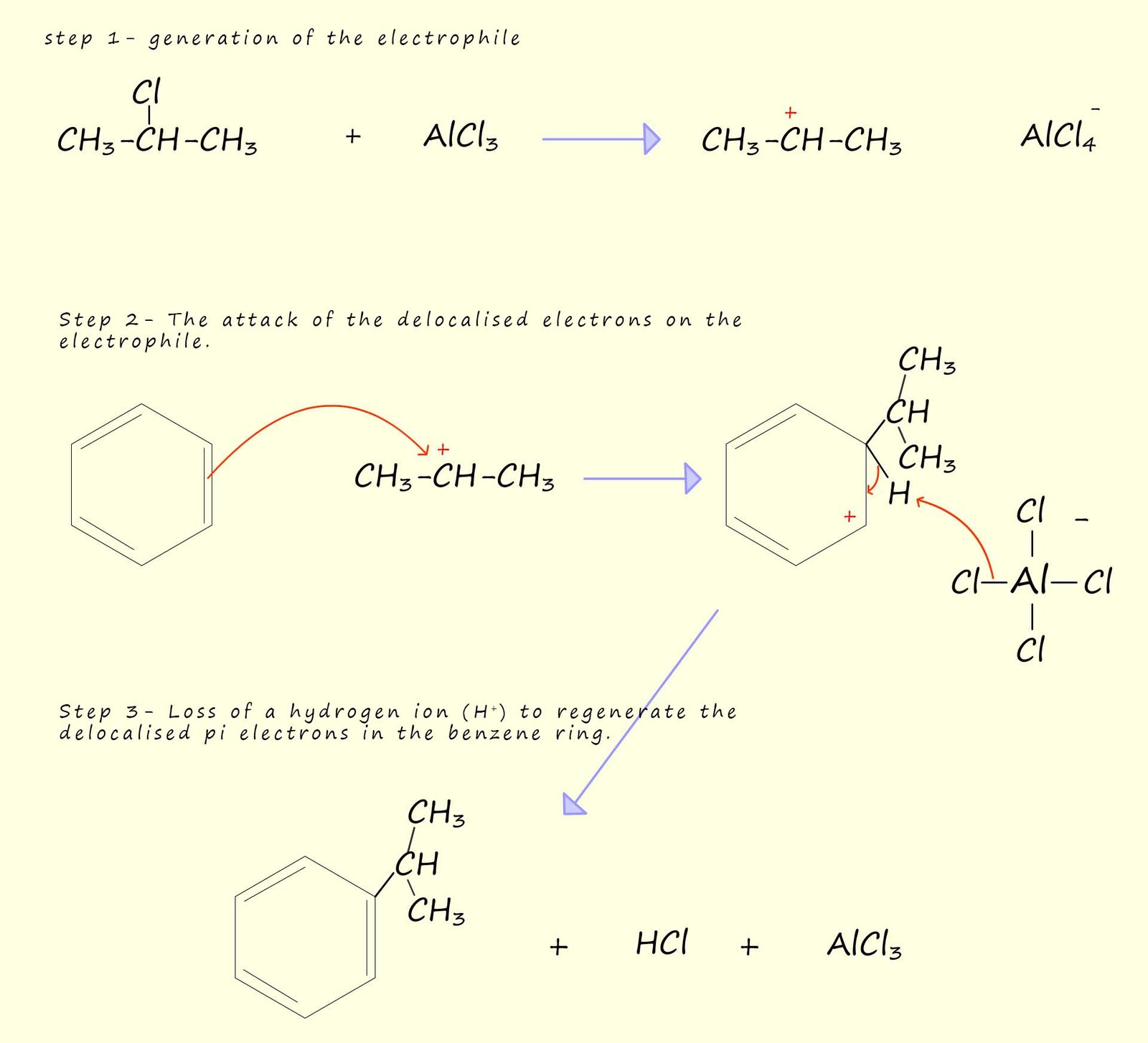
The example below shows how the industrially important chemical cumene can be synthesised in a Friedel-Crafts reaction between the halogenalkane or alkyl halide 2-chloropropane and benzene. Cumene (isopropylbenzene) is an important intermediate in the industrial production of many important chemicals including phenol and acetone (propanone). The main points to consider in this reaction; which is shown in the image below are:
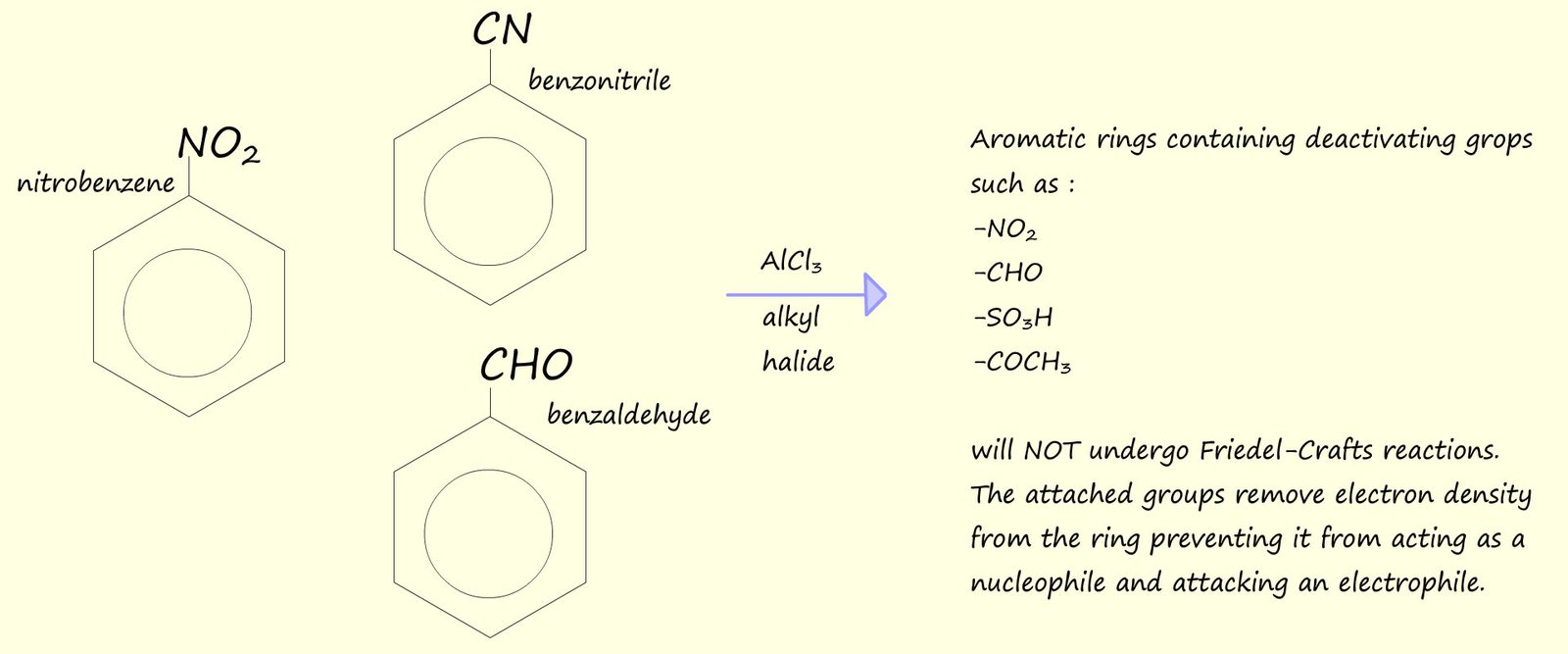
Unfortunately Friedel-Crafts alkylation reactions have a number of limitations and do not always produce the product you hoped for, some of these limitations are shown below. However there are a number of alternative routes/reactions that offer better routes to produce arenes.
One of the first mechanisms you probably learned in organic
chemistry was the electrophilic addition of hydrogen bromide and in particular the addition of hydrogen chloride to an unsaturated alkene such as ethene; as outlined below:
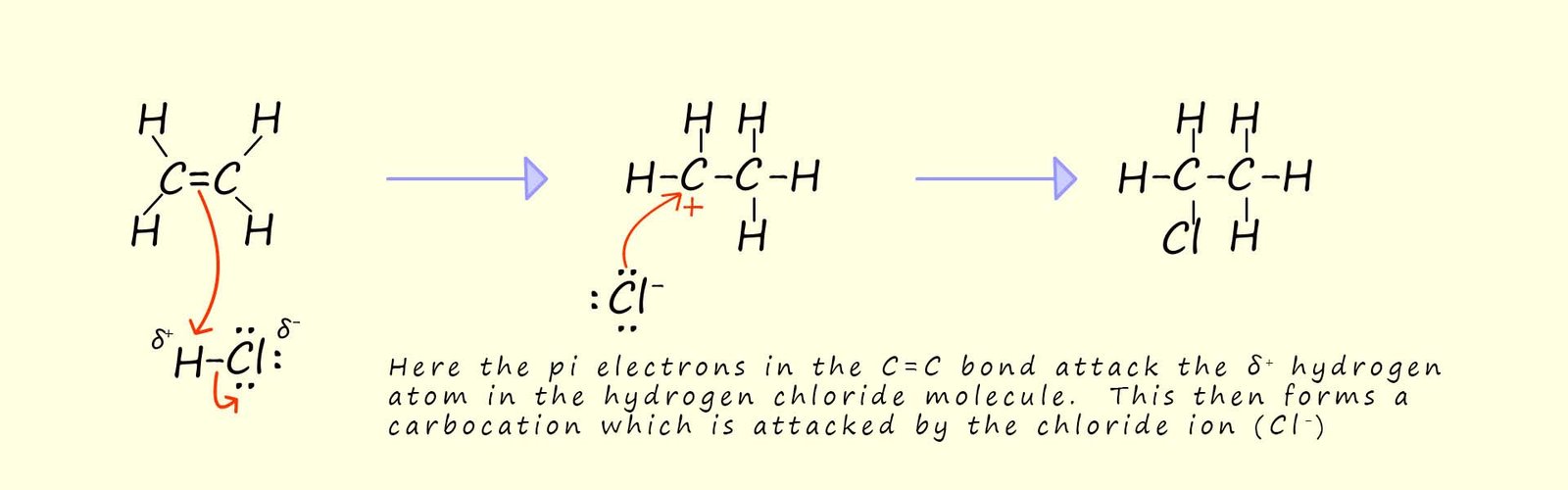
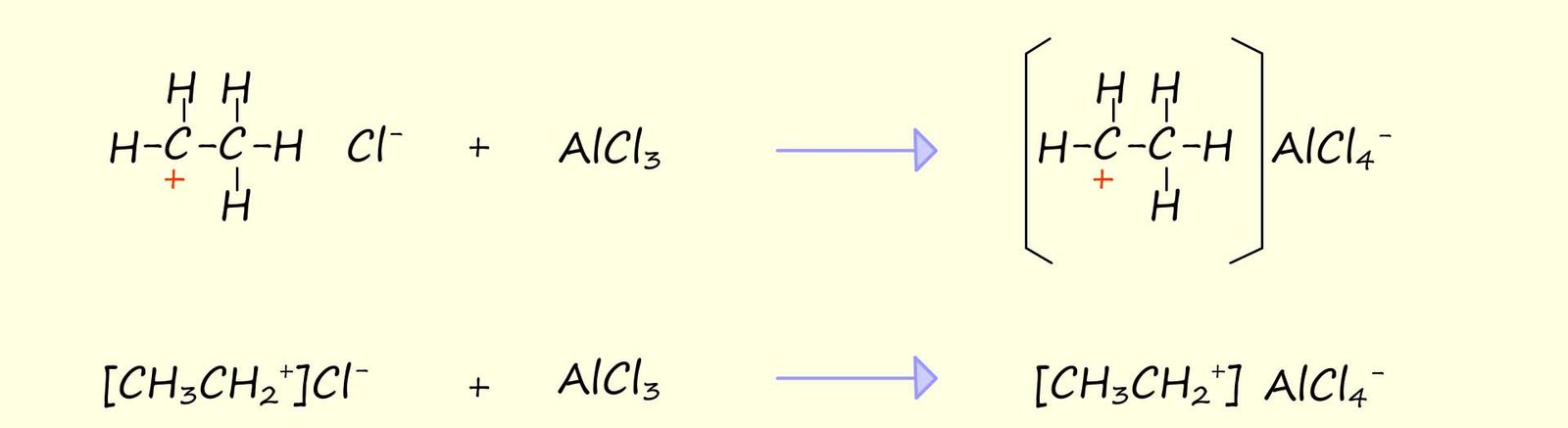
In the above mechanism the addition of the chloride ion (Cl-) to the carbocation is a relatively slow step since the chloride ion is a poor nucleophile. If a Lewis acid was added to this reaction then it could be used to "intercept the chloride ion"; this would leave the alkyl carbocation to react with something else- for example an aromatic ring! This is outlined below:
The ethyl cation which is produced in the above reaction could as mentioned be attacked by the delocalised pi(π) electrons in an aromatic ring such as benzene to form ethylbenzene, this is outlined below using both the Kekulé representation of benzene and the circle notation:
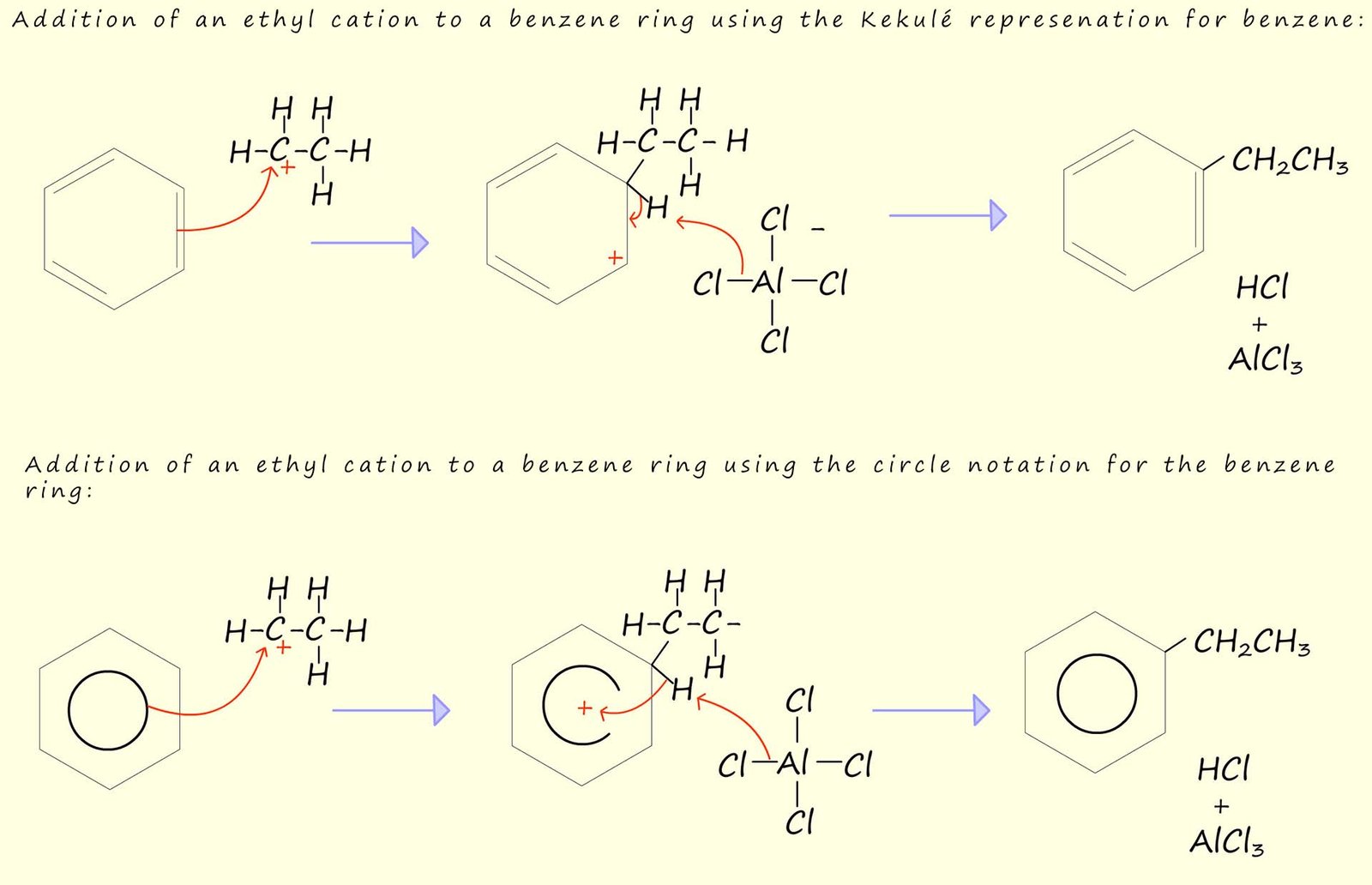
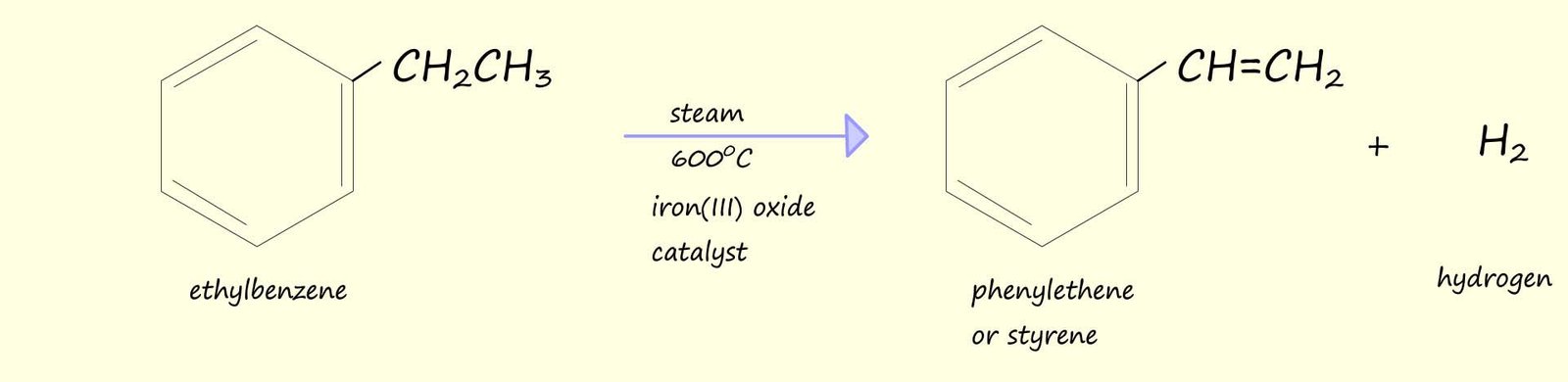
The synthesis of ethylbenzene is a particularly useful reaction since ethylbenzene can be dehydrogenated using steam and an iron(III) oxide catalyst to form phenylethene or as it is more commonly called styrene. Styrene is very useful since it is the monomer used to make the polymer polystyrene.
Friedel-Crafts alkylation reactions are not particularly useful as a general rule simply because of the limitations of this type of reaction. The main problems with Friedel-Crafts alkylation reactions are:
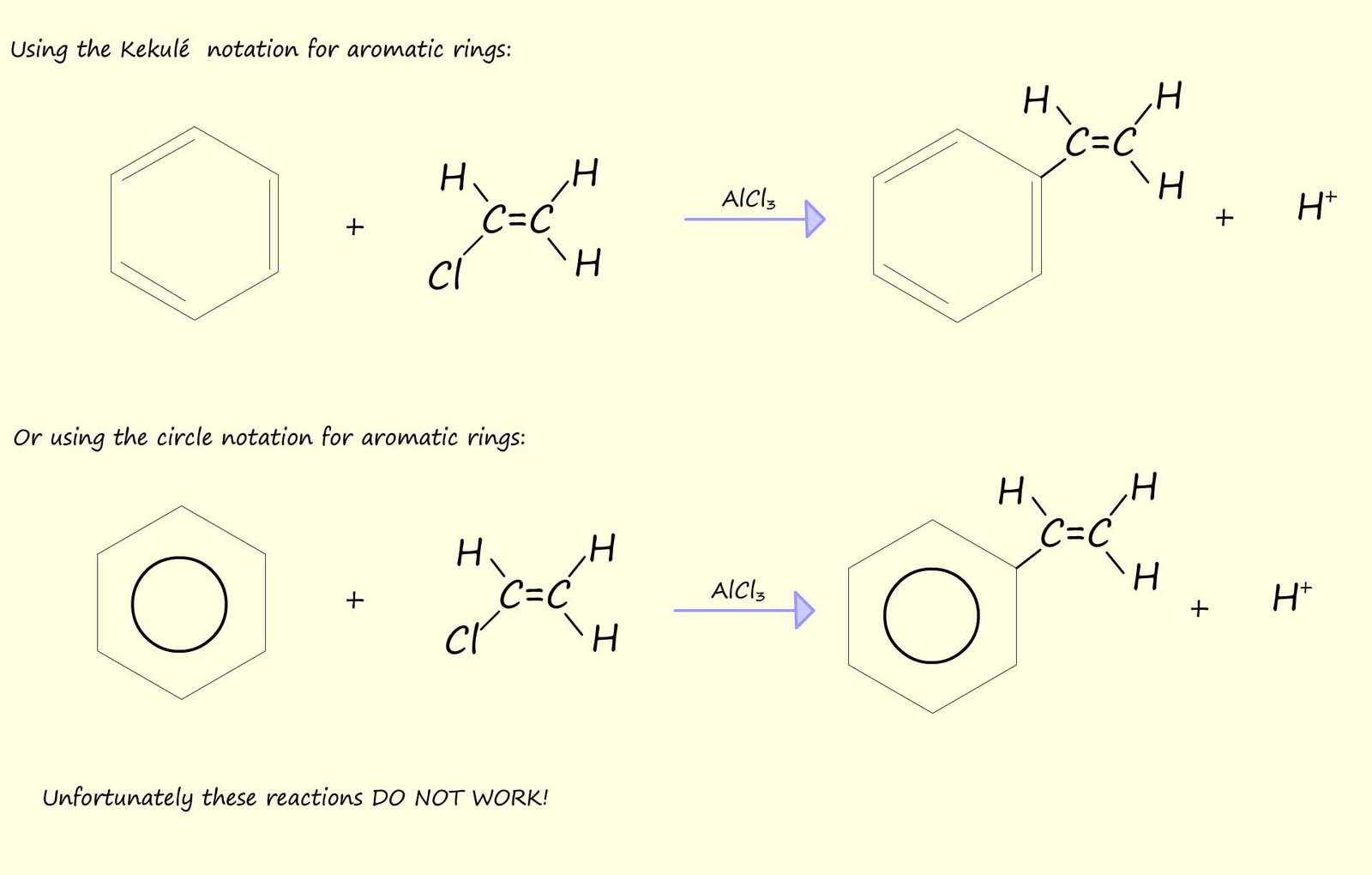
The reaction above gave one possible way in which to make ethylbenzene which was then dehydrogenated to form styrene or phenylethene. We could however imagine that styrene (phenylethene) could simply be made by the addition of chloroethene to benzene via a Friedel-Crafts alkylation reaction as shown below, however Friedel-Crafts alkylation reactions involving the addition of a vinylic group (that is a molecule which contains a C=C ) fail, they simply don't work.
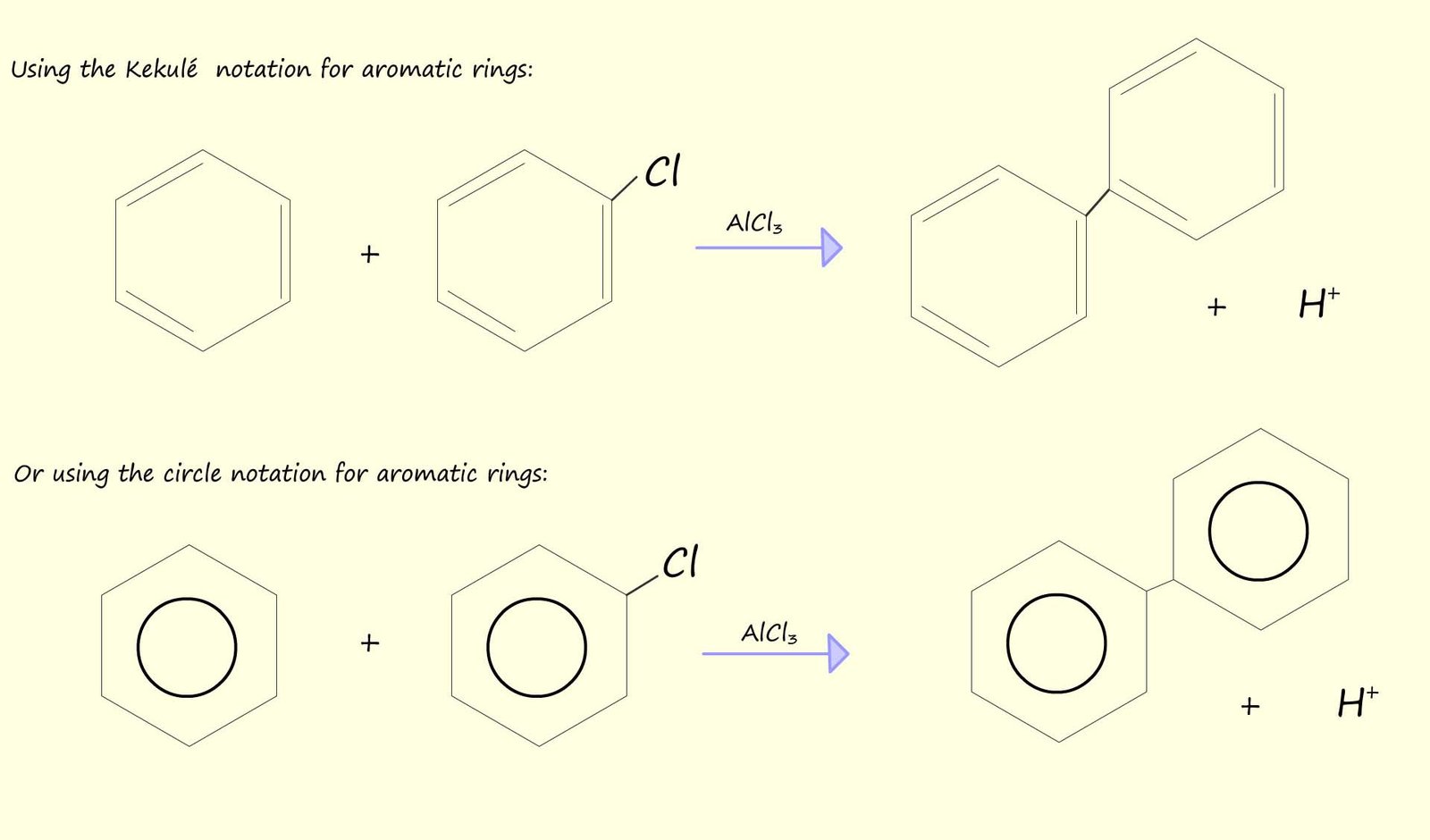
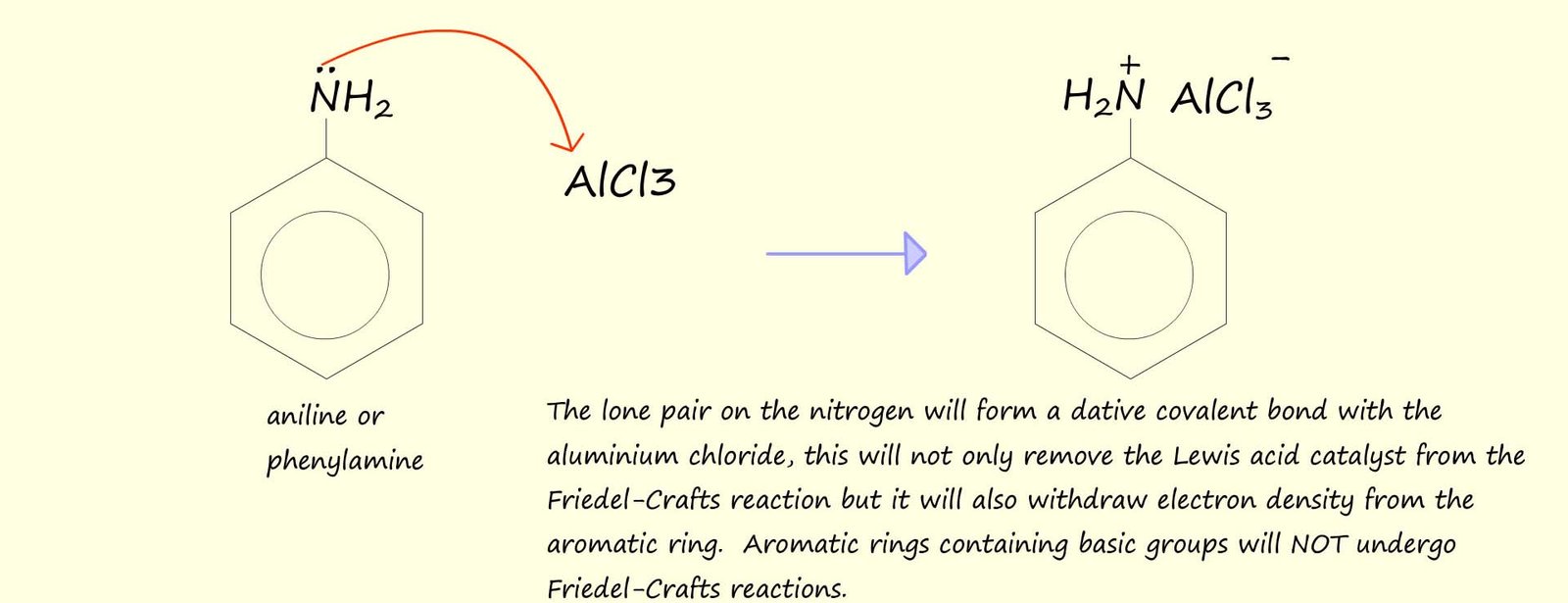
It is also worth mentioning perhaps that Friedel-Crafts reactions also fail with aryl halides, that is halides joined to an aromatic ring, this is mainly due to the fact that the C-X bond in aryl halides is much stronger than the corresponding C-X bond in a halogenalkanes or alkyl halides and also the Lewis acid catalyst, such as AlCl3, is not strong enough to effectively pull off a halogen atom from an aryl halide since this would disrupt the delocalised pi(π) electrons in the aromatic ring and result in the formation of an unstable aryl carbocation, this is a pity since this would be a very useful reaction to be able to carry out in practice since it would provide a way to build large molecules containing many aromatic rings linked together, this is outlined below:
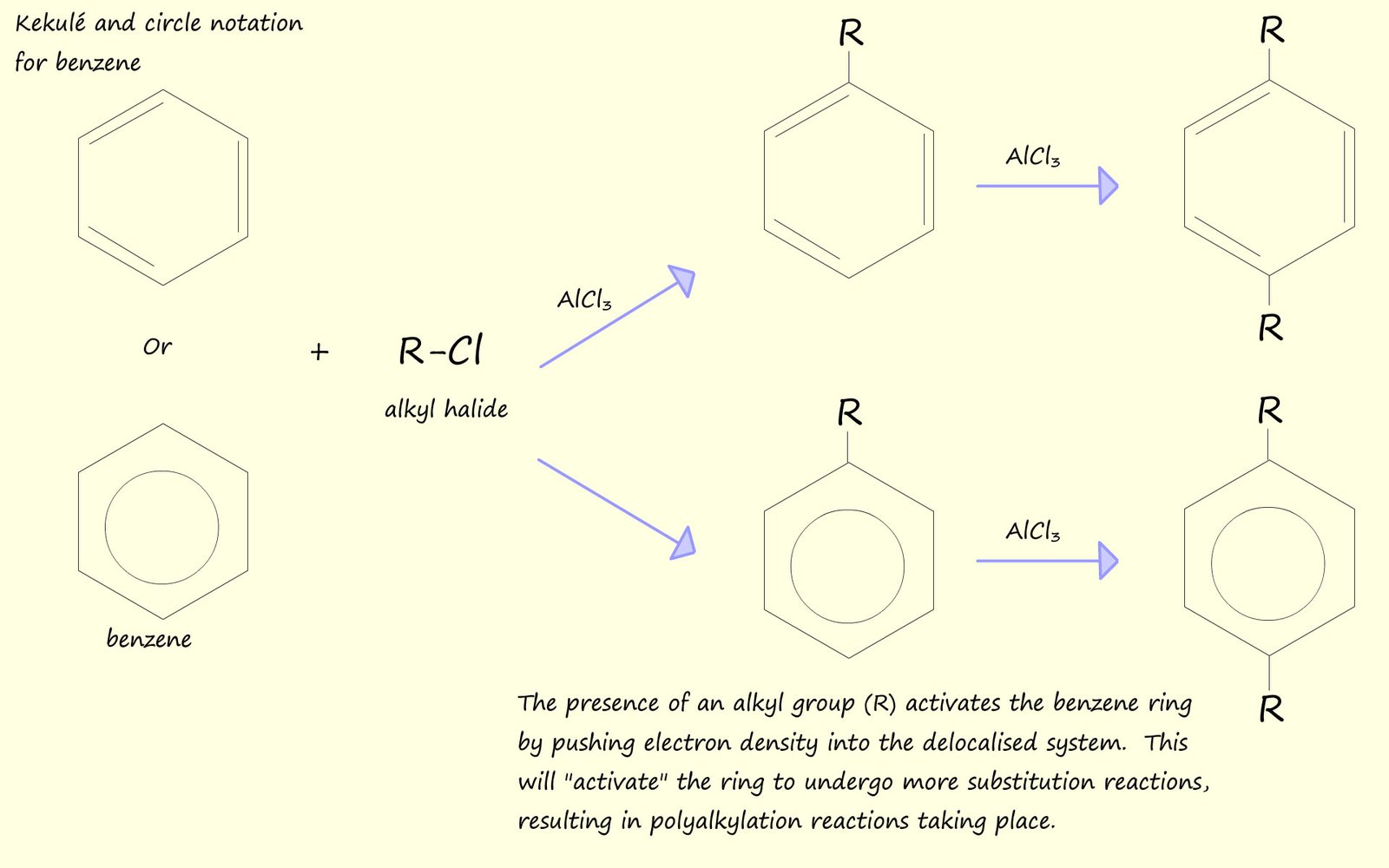
The product of a Friedel-Crafts reaction; an alkyl substituted aromatic ring or an arene is more susceptible to electrophilic attack than the starting material. This is simply because the alkyl group attached to the aromatic ring is an activating group. The alkyl group will push electrons into the aromatic ring, this makes the alkyl substituted aromatic ring much more willing to undergo further electrophilic substitution reactions which leads to polyalkylation products as shown below:
It is possible to try and reduce the possibility of polyalkylation by using a large excess of the starting reactant. However it is still likely that a mixture of polyalkylated products will be produced.
Answer the two questions below to review your understanding of a few of the main points on Friedel-Crafts alkylation reactions covered above, click the blue boxes to reveal the answers to the questions.
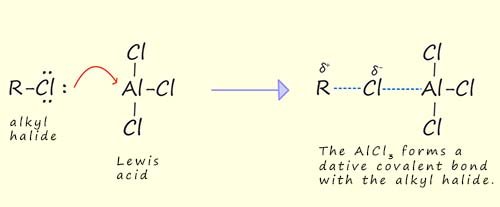
In a Friedel-Crafts alkylation reaction the electrophile is a carbocation or a carbocation like species formed by the reaction of a halogenalkane (which is also called an alkyl halide) with a Lewis acid such as aluminium chloride (AlCl3), ferric bromide (FeBr3) or born trifluoride (BF3). Recall that a Lewis acid is an electron deficient molecule that is capable of accepting a lone pair of electron from another species (a Lewis base).
Step by step explanation of how the electrophile forms:

The major disadvantages of Friedel-Crafts alkylation reactions are: