The first main use of phenol was in the 1860s where the British surgeon Joseph Lister successfully used phenol to sterilise surgical instruments and also as an antiseptic to clean wounds during surgery, however it was found to damage body tissue and this use of phenol was soon stopped. Phenol will cause blistering of the skin on contact and prolonged contact with your skin can cause deep painful burns, it can also pass through the skin and into the body where it can cause convulsions, organ damage and ultimately death. Ingestion of relatively small amounts of phenol are also likely to be fatal, bearing this in mind care is obviously needed when handling this chemical.
Today phenol is an important chemical in industry and large amounts of phenol and other compounds based on it are prepared industrially. Phenol and its compounds have a variety of uses including:

phenol based compounds are also commonly used in the :

Phenol or hydroxybenzene is a colourless white solid when pure,
but it is often appears
red or pinky in colour if impure or if it is allowed to oxidise in storage.
Phenol absorbs water from damp air and indeed phenol
crystals are often
very wet and if you ever have the opportunity to handle phenol in a lab it is
likely to be in a dissolved in water to form a solution. It has
a melting point of 40.5oC. Perhaps one
of the most distinctive properties of phenol
is its smell, I have always found it to have a rather sickly sweet smell which can linger
for a considerable time and if you ever have the misfortunate to spill
phenol in the lab it is likely you will be
smelling it for weeks to come!
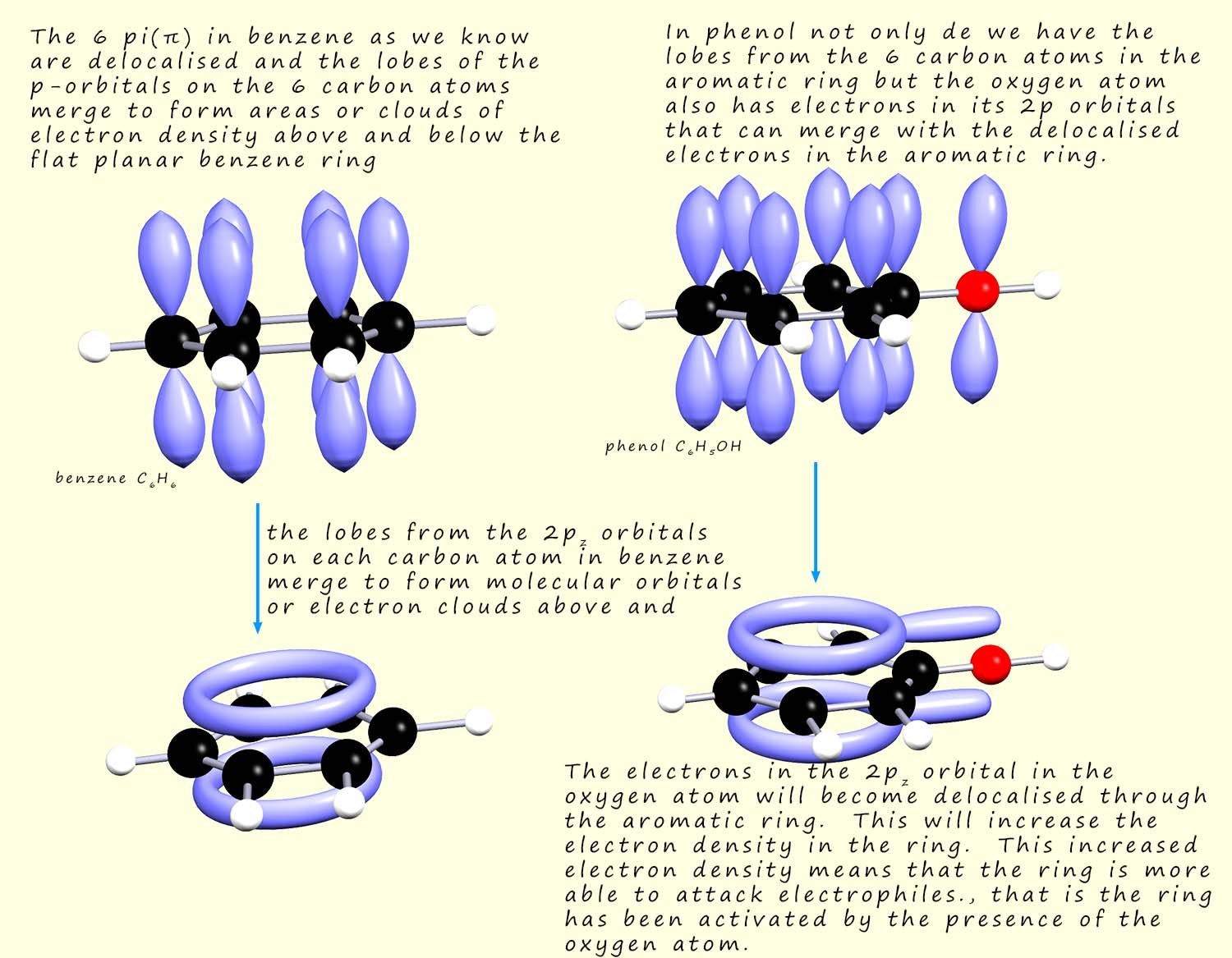
Phenol is often described as an aromatic alcohol, after all it has a hydroxyl group (R-OH) characteristic of alcohols and an aromatic ring, you might therefore predict that it will share some of the properties and reactions of alcohols and that the aromatic ring will undergo electrophilic substitution reactions. However the reactions of phenol are in some ways similar to those of alcohols but many of its reactions are quite different to those of alcohols. The aromatic ring in phenol is often described as being activated by the attached hydroxyl group (-OH). The oxygen atom attached to the aromatic ring can use a lone pair of electrons, those in the 2pz orbital and feed these into the delocalised electrons already in the aromatic ring. This will increase the electron density in the aromatic ring and make it more able to attack electrophiles. This is outlined in the diagram below:
As an example of some of the reactions of phenol consider the reaction conditions needed for the reaction of phenol with bromine and benzene with bromine:
| Reaction of bromine and benzene | Reaction of phenol and bromine |
|---|---|
| Bromine will react with benzene to form bromobenzene, this reaction requires the use of a Lewis acid catalyst, usually iron (III) bromide and liquid bromine | Phenol is dissolved in hot water and bromine water is added to the mixture, no Lewis acid catalyst is needed for a reaction to occur. |
You can clearly see that the conditions needed for the bromination of phenol are much milder than for the bromination of benzene. Phenol is simply dissolved in hot water and bromine water is added to the mixture, no Lewis acid catalyst is needed. Here the red-brown colour of the bromine water disappears immediately on addition to the phenol solution and a white coloured precipitate of 2 ,4, 6-tribromophenol is formed. The 2, 4, 6-tribromophenol is insoluble in water and smells of antiseptic. This reaction is outlined in the image below:

The equations for this reaction are shown below:

The electrophile that the activated aromatic ring in a phenol molecule attacks is a δ+ bromine atom in hypobromous acid (HOBr) which is produced when bromine dissolves in water:
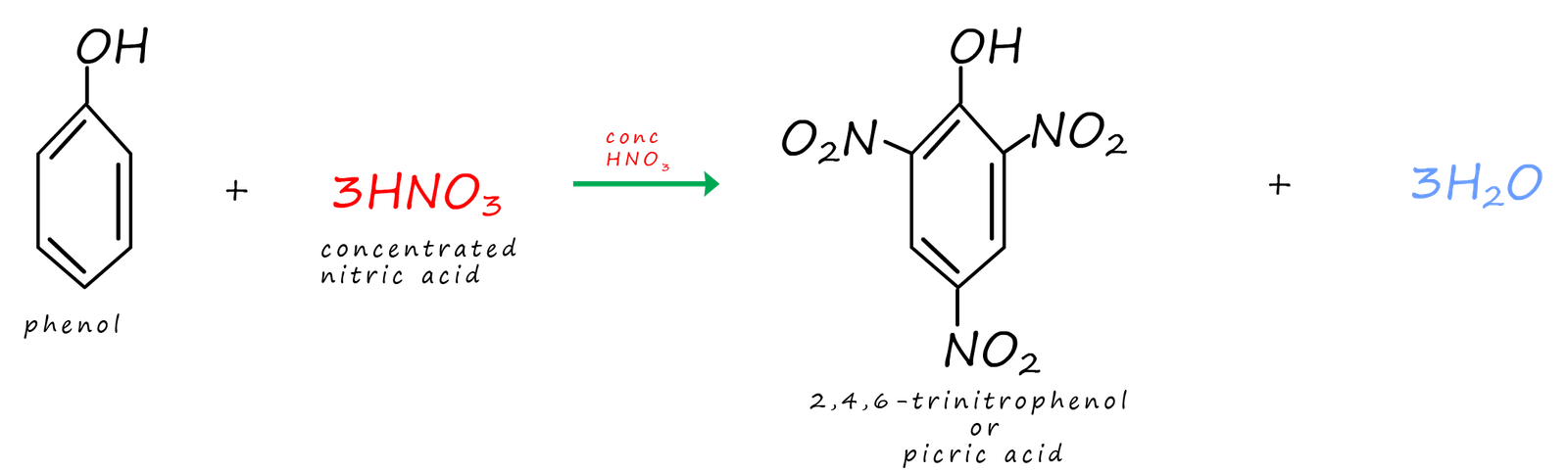
It is a similar story with the nitration of phenol. The nitration of benzene requires the use of a nitrating mixture made up of concentrated nitric and sulfuric acid but with phenol it only requires the use of dilute nitric acid. The two products of this reaction are shown below:


When nitrating phenol if the dilute nitric acid
is swapped for concentrated nitric acid then a compound called 2, 4, 6-trinitrophenol is
formed as one of the products of this reaction. This compound is often called picric acid and one of its early
uses was as a yellow dye or stain, workers who used this acid as a dye were often referred to as "canaries" because
the picric acid stained their skin bright yellow.
However perhaps the most famous use of picric acid is as a high
explosive. Experiments on the use of picric acid as an explosive were carried out in Lydd, a town in Kent. Following these experiments
picric acid was
used as a high explosive called Lyddite by the British in World War I.
Picric acid despite being an explosive is actually quite difficult to detonate especially when stored wet, which
is why it is was often mixed another explosive, TNT or trinitrotoluene which can act as a detonator.
However care is needed when storing picric acid since it will react with most metals, but not aluminium or tin, to
form metal salts which are
extremely shock sensitive and can act as detonators; which will then ignite the picric acid. This is why grenades and bombs containing picric
acid were often coated with tin. The equations below show how picric acid can form from
phenol and concentrated
nitric acid.
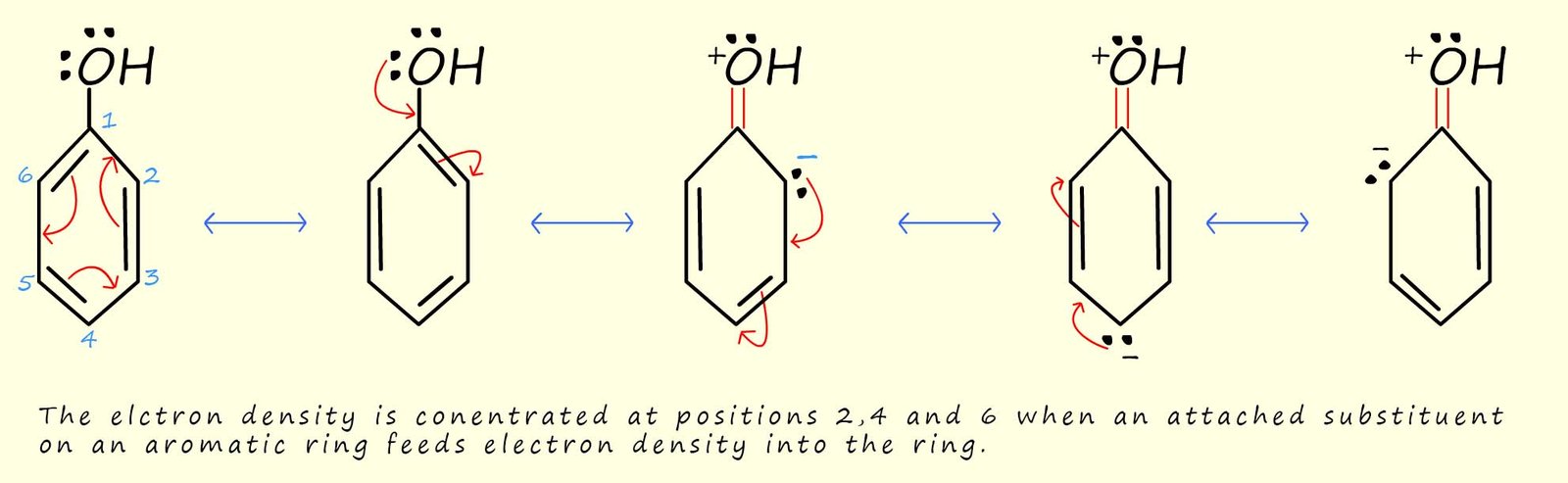
The addition of bromine atoms to the
phenol aromatic ring resulted in the formation of 2,4,6-tribromophenol. It
is no accident that the bromine atoms were added to
positions 2, 4 and 6. When a substituent is
attached to an aromatic ring the ring may become more or less reactive as a results, this was mentioned above.
If the
attached group feeds electron density into the
aromatic ring then the ring, as in the case of the hydroxyl group
in phenol then the aromatic ring is said to be activated.
This simply means that since there is a higher electron density in the ring, the aromatic ring
will be a better nucleophile and will be more able to attack an electrophile. If the attached group withdraws
electron density from the aromatic ring then we can say that
the aromatic ring will be deactivated, or less reactive
towards an electrophile.
The electron density which is feed into the aromatic ring by
any attached substituent will become part of the delocalised
electrons within the ring. We can draw a series of resonance hybrid structures to
show where this electron density is likely
to be concentrated. The diagram below shows a series of
resonance stabilised structures for an activated aromatic
ring system, such as phenol, you can see that there is a higher
electron density at positions 2,4 and 6, this
is why the electrophilic bromine atoms add to these positions.