
Phenol is often compared to alcohols
since they both contain a hydroxyl functional group (-OH), however the reactions
and properties of phenol are generally quite different and unique from
alcohols. One way in which they are both similar is
that both phenol and alcohols contain intermolecular hydrogen bonding between neighbouring molecules.

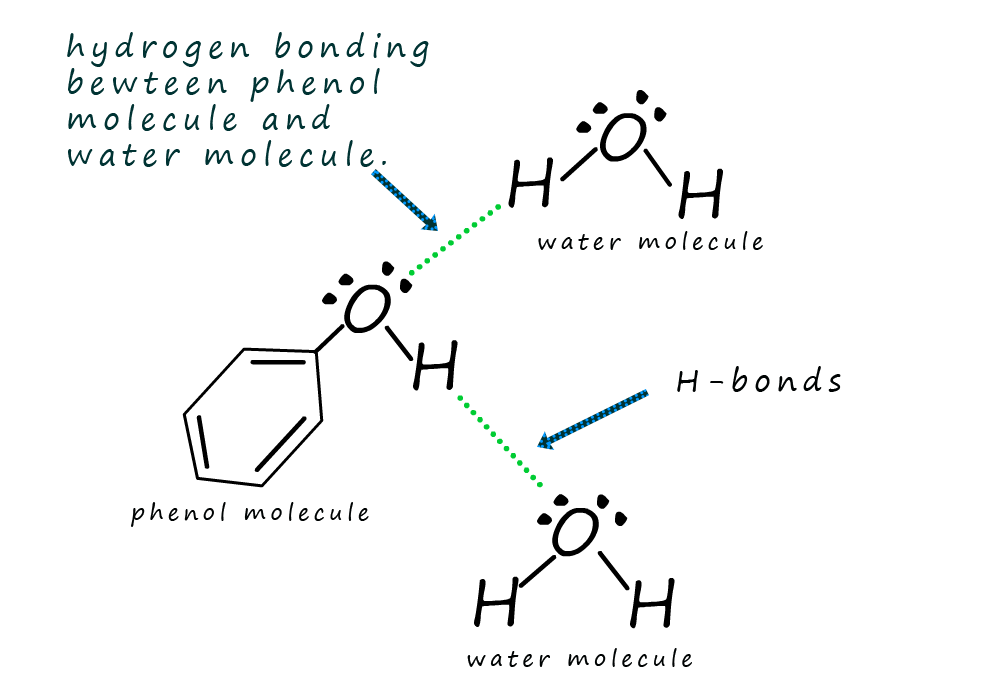
The O-H bond present in the hydroxyl functional group in phenol
is a polar one which contains a δ+ hydrogen atom
and a δ- oxygen atom. Therefore
the hydrogen atom on one phenol molecule can form
hydrogen bonds with the lone pair
of electrons on an oxygen atom in a neighbouring
molecule as shown in the diagram opposite.
The presence of this hydrogen bonding in
phenol will result in an
elevated melting and boiling point. Phenol
melts at 410C and has a boiling point of 1820C, much
higher than would be expected from such a small molecule with a low molecular mass.
Alcohols like phenol contain
a polar hydroxyl group and will therefore exhibit
similar hydrogen bonding between different alcohol molecules, the image opposite shows the hydrogen bonding present in the alcohol methanol.
When a substance dissolves there must be some sort of interaction between the solute and the solvent molecules, this
interaction is likely to be involve one or more types of intermolecular bonding. Since
phenol molecules will engage in hydrogen bonding it will show some solubility in
water at room temperature. The δ+ hydrogen
atom present in phenol will form a
hydrogen bond with the lone pairs of electrons on a
water molecule.
However a phenol molecule also contains a large
covalent phenyl group (-C6H5) attached to the hydroxyl group (-OH) and since the
bonding here is covalent you would expect this group to be insoluble in water.
So as you might expect phenol shows some
solubility in water due to the presence of
hydrogen bonding
with the water molecules, despite the presence of the large
covalent aromatic ring on the molecule.
Approximately 8.3g of phenol will dissolve in 100ml of water at room temperature. If you add more
phenol a rather
unusual phenomenon occurs, two liquid layers will form in the flask or test-tube, one layer is a solution of
phenol
in water and
the other is a solution of water in
phenol. However if these two layers are heated to
around 700C a single layer forms which is a solution phenol
in water. As previously discussed the phenol
dissolves in
water mainly due to its ability to form intermolecular hydrogen bonds
with water molecules, as shown below.
The reactions of phenol as we have mentioned above are often compared to that of alcohols, since they both contain the hydroxyl functional group (R-OH). However there are many differences in the reactions of alcohols and phenol, one area where they differ greatly is in their acidity. Phenol and many of its compounds are weak acids, while alcohols are very poor acids, for example ethanol is less acidic than water. The table below gives an indication of the relative strengths of several acids. Recall that the larger the value of Ka and the smaller the value of pKa the stronger the acid.
| acid | formula | Ka | pKa |
|---|---|---|---|
| ethanoic | CH3COOH | 1.76 x 10-5 | 4.75 |
| carbonic | H2CO3 | 4.3 x 10-7 | 6.37 |
| phenol | C6H5OH | 1.6 x -10 | 9.8 |
| water | H2O | 1 x 10-14 | 14 |
| ethanol | C2H5OH | 1.3-16 | 15.9 |
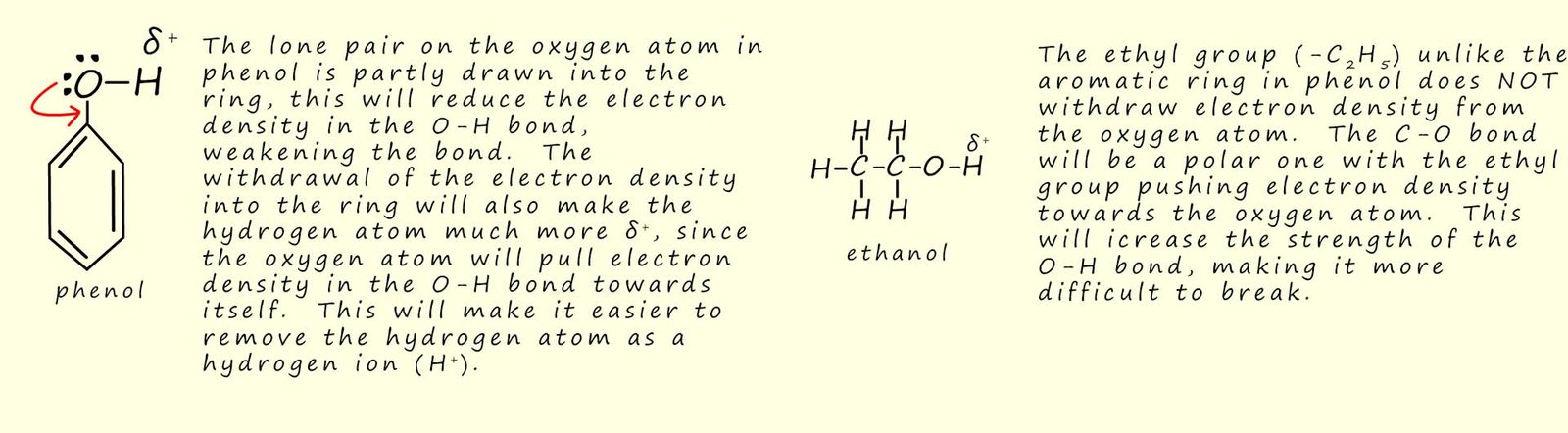
Using the pKa values in the table above we can clearly see that phenol is much more acidic than a typical alcohol such as ethanol. The hydrogen atom in the O-H bond in a phenol molecule has a much larger δ+ charge than the hydrogen atom in the hydroxyl group in ethanol due to the fact that the electrons in the lone pair on the oxygen atom in phenol are partly withdrawn into the aromatic electron delocalisation within the ring. This means that the hydrogen atom is more acidic, that is it is easier to be removed as a hydrogen ion (H+). This is outlined in the diagram below:
Recall that acidity is a property of water, so when phenol is added to water it partly dissolves to form a weak acid, often called carbolic acid. Phenol partly dissolves in water to form a hydrogen ion (H+) and a phenoxide ion (C6H5O-), this is shown in the equations below:
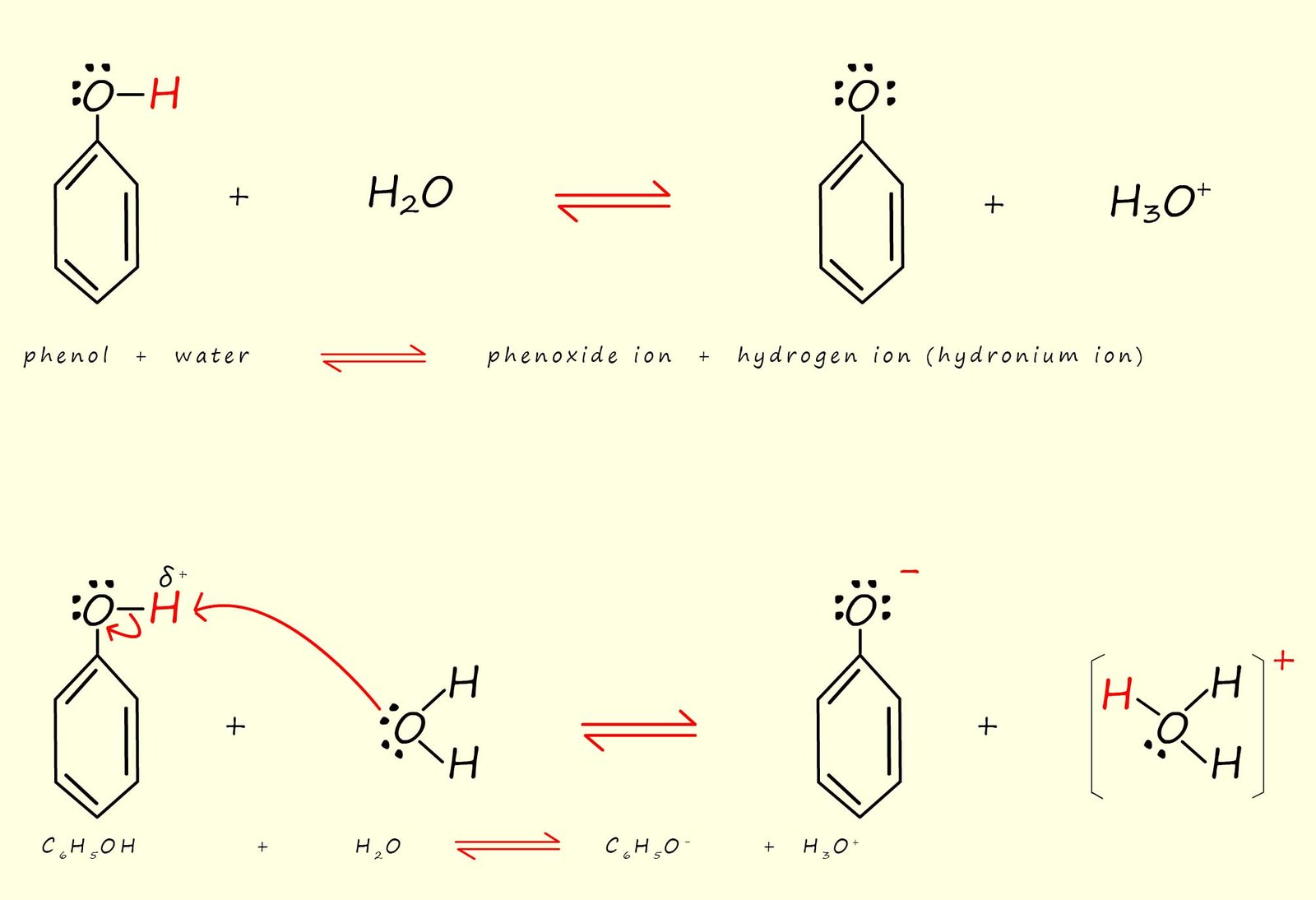
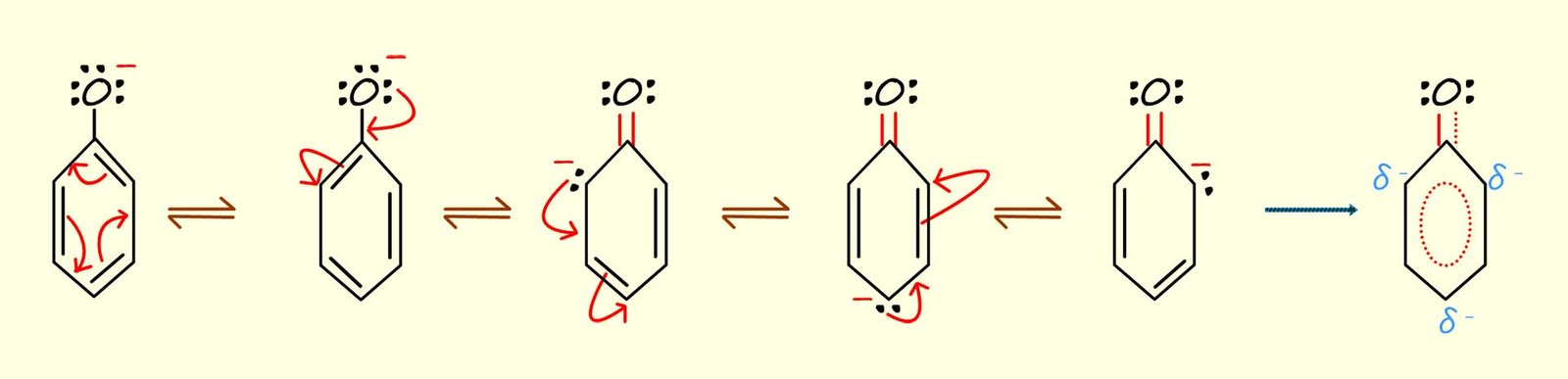
The phenoxide ion will of course be a resonance stabilised ion with the 2p electrons on the oxygen atom present in the phenoxide ion being delocalised through the aromatic ring, the image below shows each of the resonance stabilised structures for the phenoxide ion
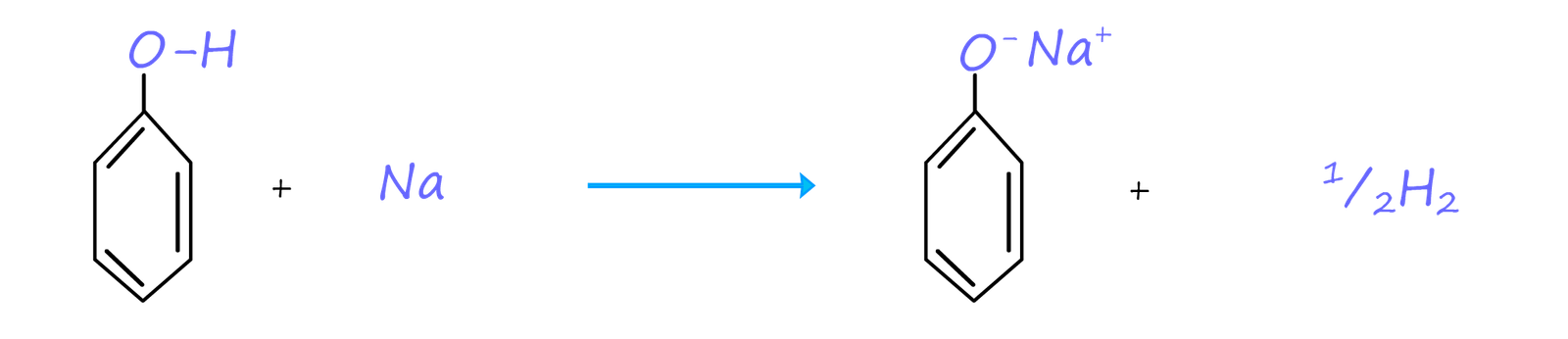
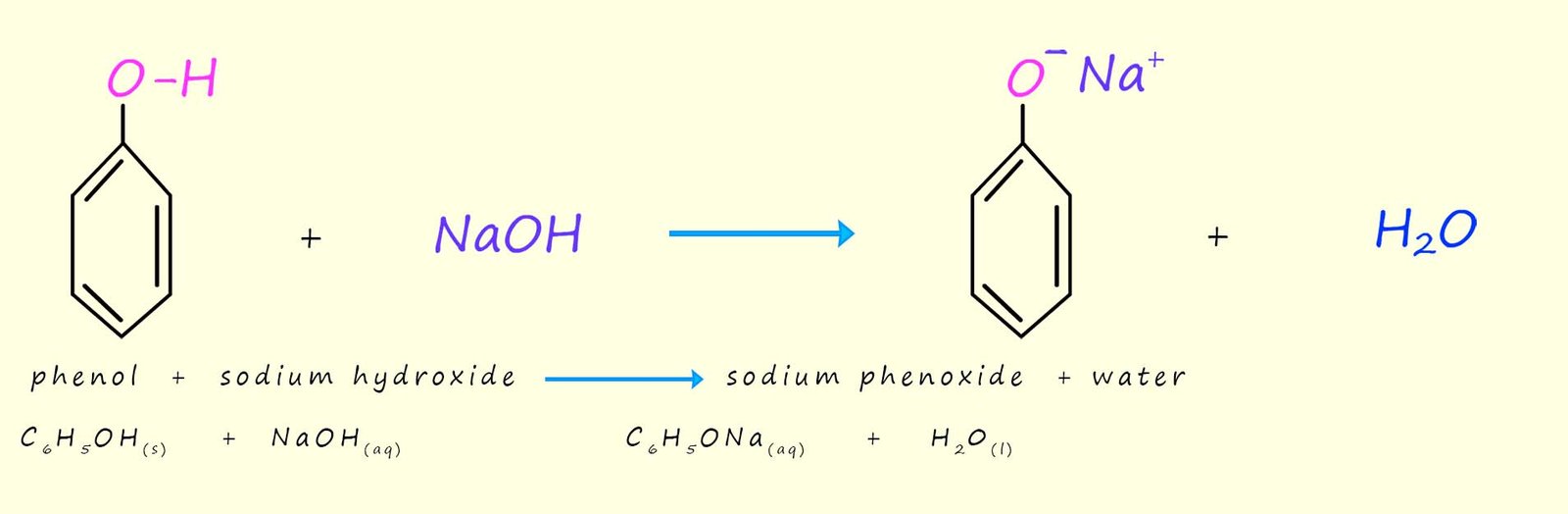
Although phenol dissolves in water to form a weak acid, it is acidic enough to undergo an acid base reaction to form a salt and water. So while phenol is only slightly soluble in water it readily reacts with alkalis such as sodium hydroxide to form soluble salts e.g. the image below gives equations for the acid-base reaction of phenol with the strong alkali sodium hydroxide to form the soluble salt sodium phenoxide and water.
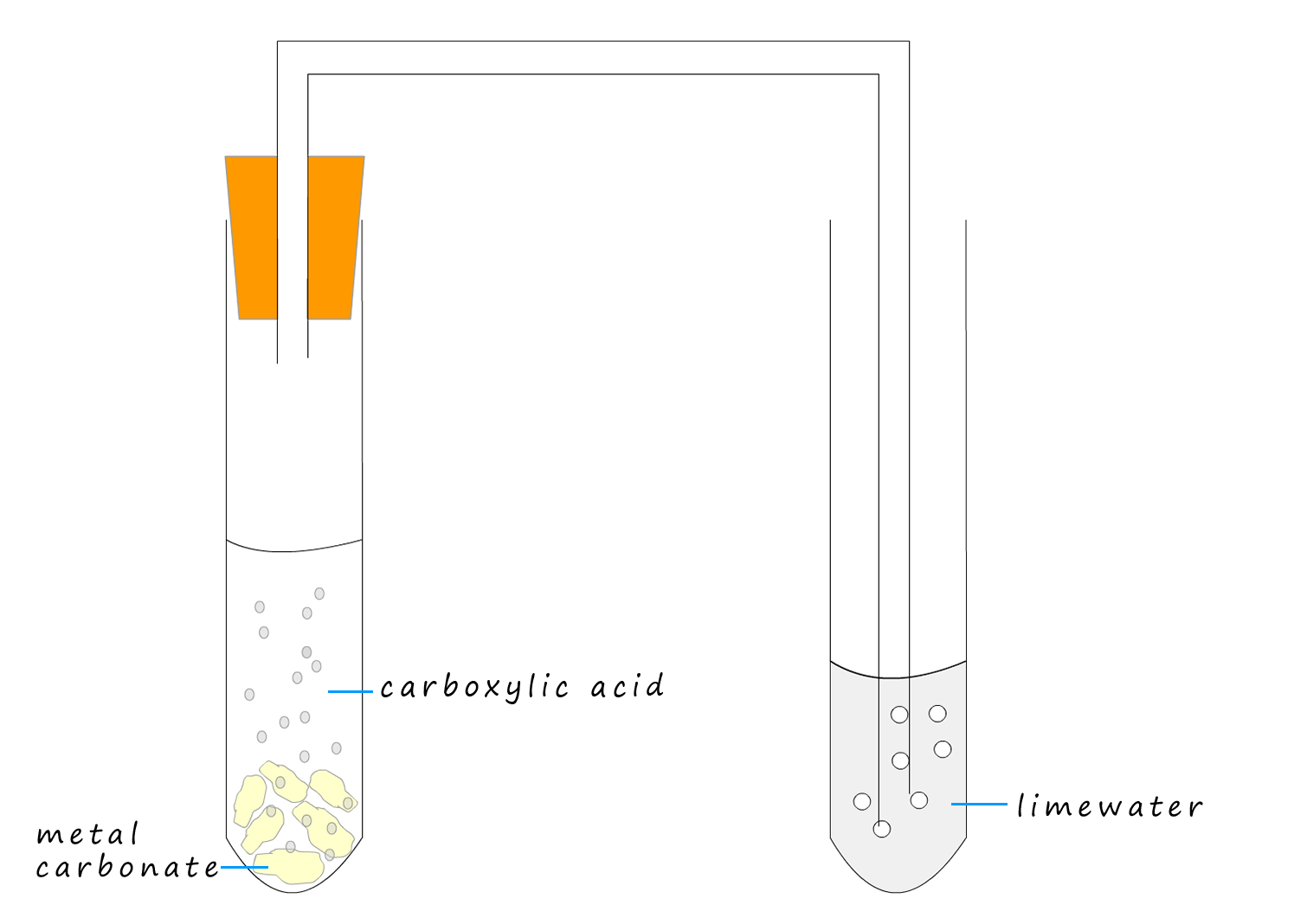
A reaction you have seen before is the reaction between an acid and a metal carbonate such as sodium carbonate or calcium carbonate to form a salt, water and carbon dioxide gas, an equation for this reaction is shown below:
Indeed this reaction is often used as a test for acidity. This simple test is used with both mineral acids such as hydrochloric and sulfuric acids as well as carboxylic acids. However while minerals acids (pKa < 1) and carboxylic acids such as ethanoic (pKa =4.75) are sufficiently acidic to react with metal carbonates and solutions of carbonates to release carbon dioxide gas phenol is a much weaker acid (pKa= 9.8) and it is not able to produce carbon dioxide gas from metal carbonates.
As an example consider the reaction of sodium metal with alcohols. This reaction is very similar to that of sodium with water. At first glance this may seem odd as alcohols and water appear to have very different structures. However all the parts of the alcohol molecule circled in the image below do not take part in any of the chemical reactions of the alcohol molecules, only the hydroxyl functional group (-OH) group takes part in its reactions.
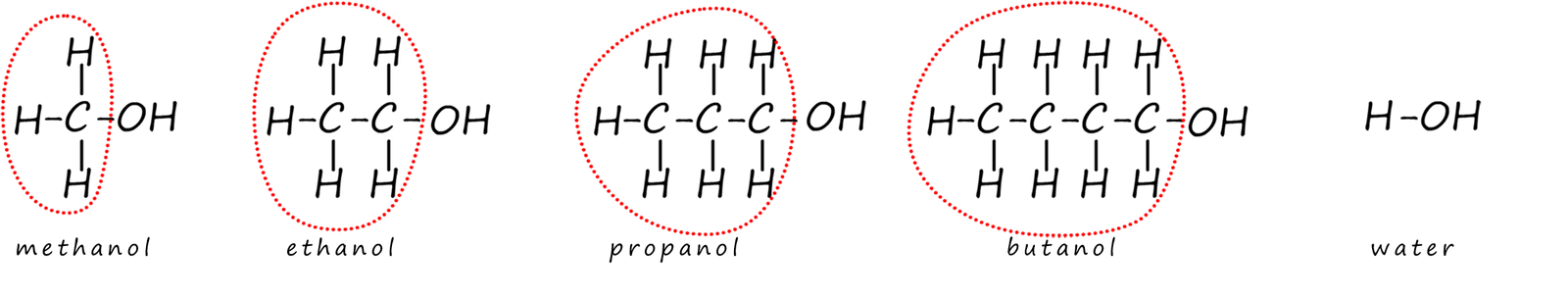
So if you swap all the "circles groups" and simply replace them with an alkyl group (-R) then we have:

With the alkyl groups on the alcohol molecules replaced by a -R group it is very easy to see how similar alcohols and water are.
Consider the reaction of sodium metal with water, equations for this reaction are shown below:
Well you can think off the hydroxide ion (OH-) in the sodium hydroxide produced in the above equation as simply a water molecule (H2O), or H-OH which
has lost a hydrogen, this lost hydrogen is given off as hydrogen gas during the reaction.
When sodium reacts with alcohols the same reaction occurs. The sodium removes the hydrogen attached
to the hydroxyl functional group in the
alcohol. The lost hydrogen is given off as hydrogen gas:
These equations are all very similar the alcohol loses its hydrogen from the -OH group and it is replaced by sodium- that’s all there is to it!
As mentioned above the structure of an alcohol can be considered in some ways similar to that of a water molecule. However as the chain length of the alcohol grows then the rate of reaction of the alcohols with sodium begins+ to slow down. Methanol (CH3OH) is the smallest alcohol and it reacts the fastest with sodium metal. Ethanol (C2H5OH) the next alcohol reacts more slowly with sodium and propanol the next alcohol in the homologous series reacts even more slowly. This is shown in the image below where the rate of release of hydrogen gas gives an excellent indicator of the speed of the reaction taking place.

Phenol being a weak acid will react with alkali metals to give, as you might expect a salt and hydrogen gas. Since phenol is a weak acid this reaction is slow. It is usually carrried out by placing a small amount of solid phenol in a boiling tube and warming it gently until the phenol melts forming a liquid. A small piece of sodium or potassium can then be added to the molten phenol. The salt formed will be a phenoxide e.g.