

Higher and foundation tiers
Let's assume you have a mixture of three liquids in a flask. These liquids are water, which boils at 1000C, ethanol (alcohol) which boils at 780C and acetone (nail varnish remover) which boils at 560C. To separate a mixture of these liquids a process called fractional distillation is used. If all that is needed is to separate a liquid from a solution such as a salt or sugar solution then simple distillation can be used, this is similar to fractional distillation but without the need for a fractionating column.
Distillation is simply evaporation followed by condensation and by carefully monitoring temperatures separating a mixture of liquids with different boiling points is very easy. The image below shows the apparatus set-up in the lab to carry out fractional distillation.
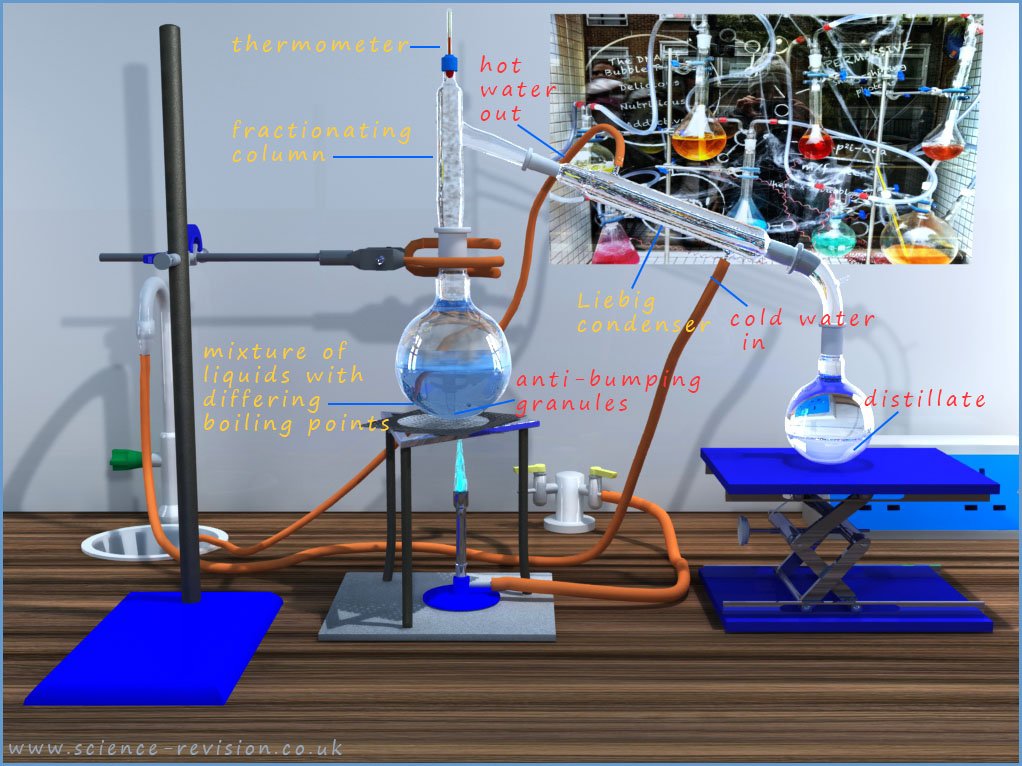
The mixture of the three liquids to be separated is placed in a round bottomed-flask and heated gently with a Bunsen burner or an electrical heater A few anti-bumping granules, usually some pieces of broken ceramic pot or small plastic beads are added to the mixture of liquids. The anti-bumping granules will ensure the mixture of liquids boil smoothly and help prevent large gas bubbles forming which can cause splashing and bumping in the mixture.
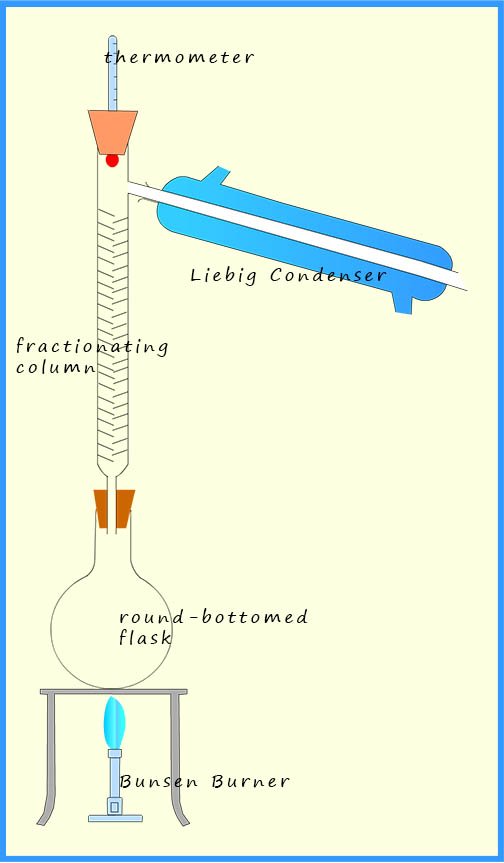
As the mixture of liquids is heated the liquid with the lowest boiling point will evaporate first, in this case it is the acetone, with a boiling point of 560C. The acetone vapour will rise up the flask and enter the fractionating column. This is simply a long glass tube which is usually filled with glass beads or balls or it may have lots of small glass spikes sticking out from its sides. The fractionating column has a temperature gradient; it is hot at the bottom and gets cooler the higher you go up the column. Now the acetone vapour will rise up the fractionating column until it hits glass beads cooler than 56oC, the boiling point of the acetone, at which point it will condense on the glass beads and the liquid acetone formed will start to fall back down into the round bottomed flask.
The same will happen to the ethanol and the water vapours, but since these two liquids have boiling points higher than the acetone the ethanol and water vapours will not rise as far up the fractionating column before they condense and turn back into liquids. The temperature in the fractionating column should be adjusted so that the acetone vapour is condensing near the top of the fractionating column with the ethanol vapour condensing around the middle of the column and the water vapour or steam condensing near the bottom of the column. Essentially what is happening is that the fractionating column has separated out the three liquids from the mixture, so if the temperature is increased slightly then each of the vapours will rise further up the column and enter the Liebig condenser one at a time where they will condense and can be collected separately.
Now the Liebig condenser is simply a tube within a tube; the inner tube is kept cool by a constant stream of cold water from a tap. The water then leaves the Liebig condenser at the top, as shown in the simplified diagram opposite. The first vapour to enter the Liebig condenser will be the acetone, when this is happens the thermometer in the still head will read 560C, the boiling point of acetone. As the acetone vapour hits the cool inner Liebig tube it will condense and turn back into liquid acetone, which can be simply collected in a beaker, flask or test-tube.
When the temperature on the thermometer begins to rise above 560C then all the acetone has been collected and the next volatile liquid, in this case the ethanol, boiling point 780C will start to enter the Liebig condenser where it will cool and condense to form liquid ethanol. Now simply replace the collecting beaker with a new one and you can collect the liquid ethanol. The ethanol vapour as with the acetone vapour will enter the Liebig condenser and condense back into liquid ethanol. Once again when the thermometer reading starts to rise above 780C then a new beaker is required to collect the next liquid, in this case the steam or water vapour. The thermometer this time will read 1000C as the water is about to enter the Liebig condenser.
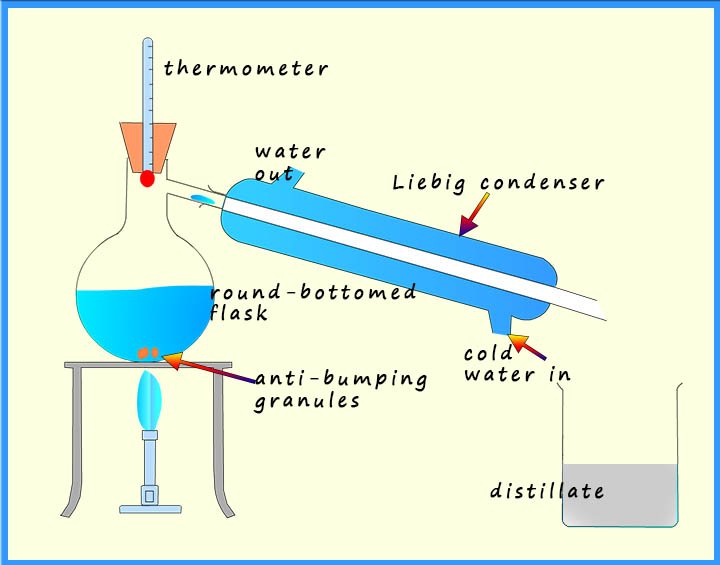
If all that is required is to separate a liquid from a solution, for example separating out water from a salt water solution then simple distillation can be carried out. This is simply the same set-up as fractional distillation above but without the fractionating column. The set-up for simple distillation is shown opposite.
Here the salt water is placed in the round-bottomed flask along with a few anti-bumping granules. When the solution is heated the water will evaporate and enter the Liebig condenser where the water vapour or steam will condense and the liquid water formed can be collected. The solid salt will be left in the round-bottomed flask.
Match the terms below with the correct defintions by simply clicking on the term and then its correct defintion. Correct responses will turn the defintions green!
Use the flashcards below to test your understanding of the key ideas on this page: