

When a chemical reaction happens energy is either given out or taken in. In exothermic reactions heat energy is released to the surroundings; whereas in endothermic reactions heat energy is absorbed from the surroundings. This energy change for a reaction is called the enthalpy change (ΔH).
In calorimetry we work out the enthalpy change indirectly by measuring how much the temperature of a substance; usually water or a solution changes during a reaction. If the water warms up it must have gained heat energy and if it cools down it must have lost it. By measuring this temperature change (ΔT) we can calculate how much heat energy was transferred. This method of measuring heat transfer is called calorimetry and the equipment used is called a calorimeter.
When substances are heated the particles they are made of gain kinetic energy, so their temperature rises. However if you supply the same amount of heat energy to different substances you will find that their temperature does not increase by the same amount. Some substances warm up quickly; while others heat up much more slowly.
This difference is explained by a property called specific heat capacity (symbol c). It tells us how much heat energy is needed to raise the temperature of 1 g of a substance by 1 K. The units are J g-1 K-1 (or equivalently J kg-1 K-1).
For example the specific heat capacity of water is 4.18 J g-1 K-1. This means it takes 4.18 J of heat energy to raise the temperature of 1 g of water by 1 K (or by 1 °C). If you choose to work in kJ kg-1 K-1; then the mass must be in kilograms (kg) and the energy in kilojoules (kJ).
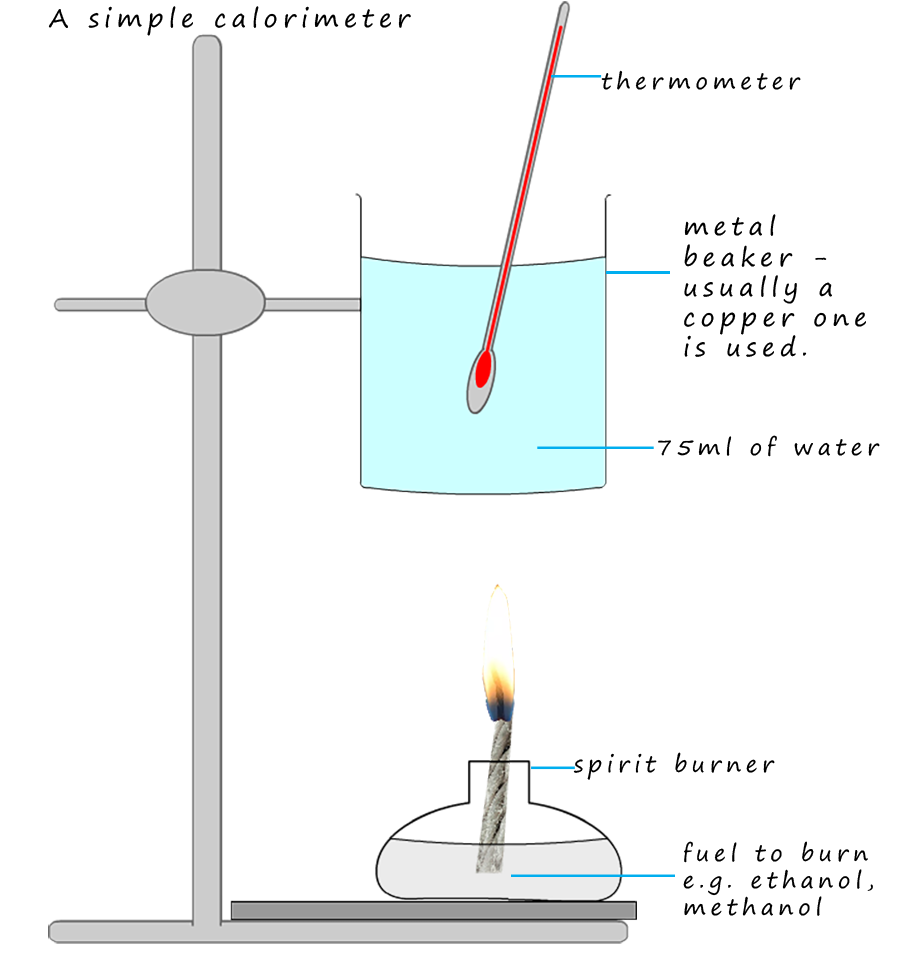
Burning fuels is an exothermic reaction. We can use a simple calorimeter like the one shown opposite to calculate the enthalpy of combustion of a fuel such as ethanol or methanol. We can use the equation below to calculate the amount of heat energy that is transferred to the water by the burning fuel:
Where:
1 g of ethanol (C2H5OH) was burned in a spirit burner. The burner was used to
heat 100 g of water in a beaker.
The initial temperature of the water was 25 °C and the final
temperature was 70 °C. Calculate the enthalpy change for this
combustion reaction.
To calculate the enthalpy change we substitute the values into the equation provided above. Care must be taken with
units, as this is the most likely cause of an error. We will make a very large assumption here that all the heat
energy from the burning ethanol is transferred to the
water. So we have:
If all the heat energy lost by the burning ethanol is transferred to the water; then the ethanol loses 18.8 kJ of energy and the water gains 18.8 kJ of energy. Now recall the first law of thermodynamics; which states that energy cannot be created or destroyed. In reality using our simple calorimeter it is very unlikely that all the heat energy from the burning ethanol is transferred to the water, as a lot of heat energy will be lost to the surroundings.
We can however extend the calculation above to work out the enthalpy change per mole of ethanol by using the formula:
The Mr of ethanol (C2H5OH) is 46, so the mass of
1 mole of ethanol is 46 g. In the example above 1 g of
ethanol was burned and this gave an enthalpy change of -18.8 kJ.
So the number of moles of ethanol present in 1 g is:
To calculate the molar enthalpy change (heat energy released by burning 1 mole of ethanol) we use the formula:
The actual enthalpy of combustion of ethanol is
-1367 kJ mol-1.
The fact that this is much more exothermic (more negative) than the value calculated above should not be a total surprise since you can probably
spot many ways in which heat from the burning alcohol is lost and not transferred
into the water. We assumed that all the heat energy from the burning
alcohol flame was transferred into the water. This is clearly not the case.
The main area of heat loss is likely to be:
However, other areas of potential heat loss are:
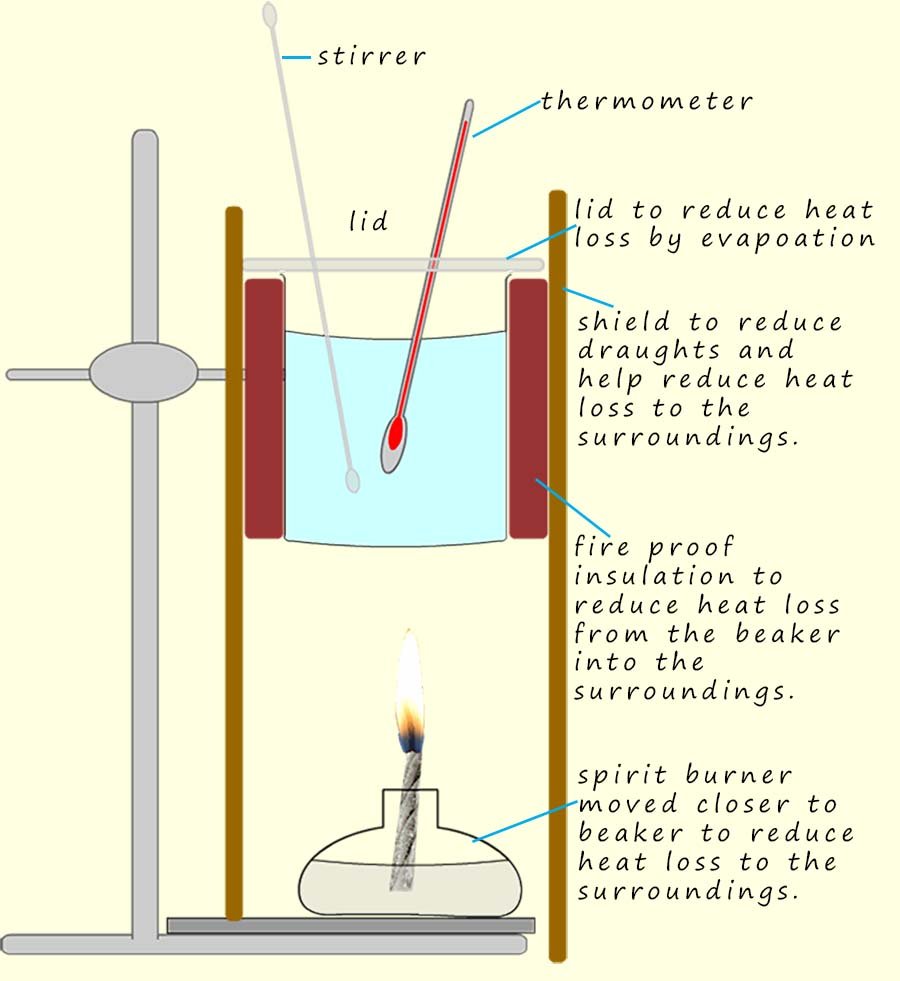
However, there are a number of changes that can be made to this simple calorimeter to help reduce heat loss and improve the accuracy of the calculated enthalpy change. These changes are summarised in the diagram opposite:
Answer the four questions below to review your understanding of the main points covered above.
Answer each question, then click an option to check instantly.
1) In a combustion experiment, the q value you calculate using q = m x c x ΔT is for the water. Is q positive or negative?
2) For the combustion reaction itself, is the ΔH value positive or negative?
3) If heat is lost to the surroundings, what happens to the ΔH value you calculate from the temperature rise of the water?
4) In the equation q = m x c x ΔT, can the temperature change be in K or in °C?